NDC Code(s) : 17518-050-01, 17518-050-02, 17518-050-00
Packager : Solventum US OpCo LLC
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| 3M Avagard DAlcohol LOTION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Solventum US OpCo LLC(006173082) |
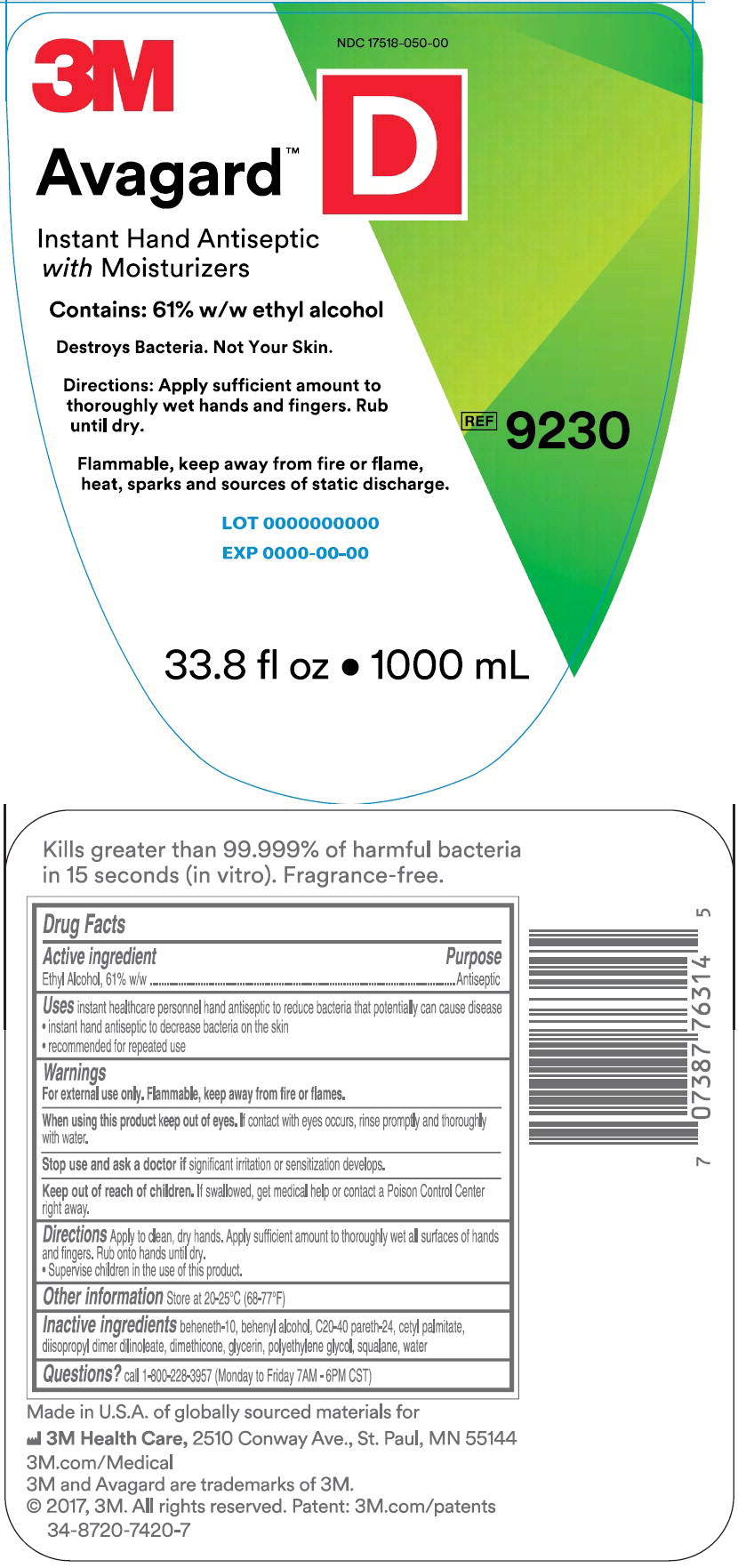
PRINCIPAL DISPLAY PANEL
NDC 17518-050-00
3M
Avagard™ D
Instant Hand Antiseptic with Moisturizer
Contains: 61% w/w ethyl alcohol
Destroys Bacteria. Not Your Skin.
Directions: Apply sufficient amount to thoroughly wet hands and fingers.
Rub until dry.
Flammable, keep away from fire or flame, heat, sparks and sources of static discharge
REF 9230
33.8 fl oz • 1000 mL