NDC Code(s) : 17518-051-01, 17518-051-04
Packager : Solventum US OpCo LLC
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
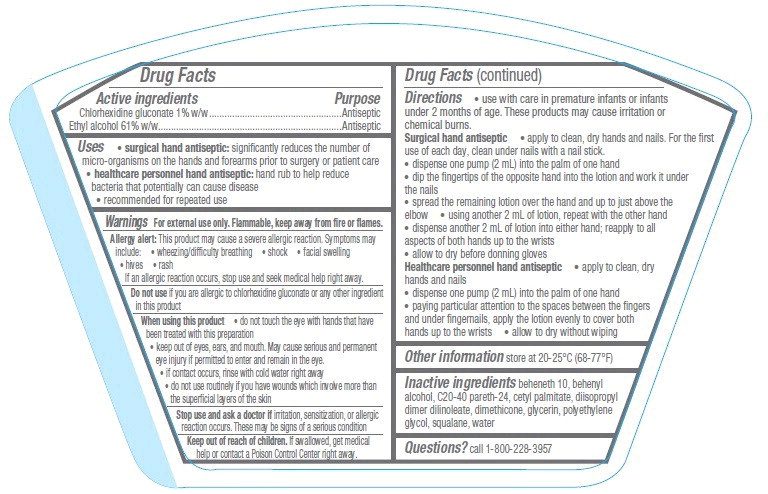
INGREDIENTS AND APPEARANCE
| 3M AvagardChlorhexidine Gluconate and Alcohol LOTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Solventum US OpCo LLC(006173082) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Grain Processing Corporation | 198484180 | API MANUFACTURE(17518-051) | |
PRINCIPAL DISPLAY PANEL
3M
NDC 17518-051-01
Avagard™
(Chlorhexidine Gluconate 1% Solution and Ethyl Alcohol 61% w/w)
Surgical and Healthcare Personnel Hand Antiseptic with Moisturizers
Peel to read Drug Facts Panel before use
Made in U.S.A. for 3M Health Care
2510 Conway Ave., St. Paul, MN 55144
1-800-228-3957
www.3M.com/infectionprevention
© 2017, 3M. All rights reserved. The shape and colors of the bottle and wall bracket are trademarks of 3M.
Patent: 3M.com/Patents
MAL10110039XC
Flammable, keep away from fire or flame, heat, sparks and sources of static discharge.
LOT 0000000000
EXP 0000-00-00
16 fl oz • 500 mL
REF 9200