NDC Code(s) : 17518-054-01
Packager : 3M Company
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| 3M Nexcare Cold Sore Treatment3M Nexcare Cold Sore Treatment OINTMENT | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
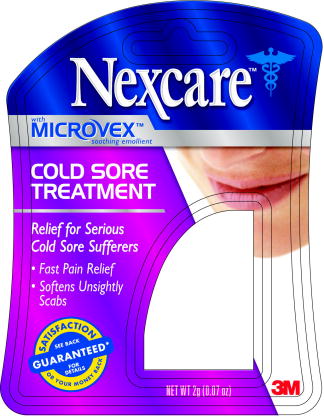
PRINCIPAL DISPLAY PANEL
Package Label
1 Tube
NET WT 2g (0.07 oz)
3M™ Nexcare™ Cold Sore Treatment
Benzocaine 5%
Allantoin 1 %
Skin Protectant
OTC Monograph
3M Consumer Health Care, 3M Center, St. Paul, MN 55144-1000
3M™ Nexcare™ Cold Sore Treatment - NET WT 2g (0.07 oz)