NDC Code(s) : 17518-055-02, 17518-055-01, 17518-055-00
Packager : Solventum US OpCo LLC
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| 3M Avagard Foaming Instant Hand AntisepticAlcohol LIQUID | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LABELER - Solventum US OpCo LLC(006173082) |
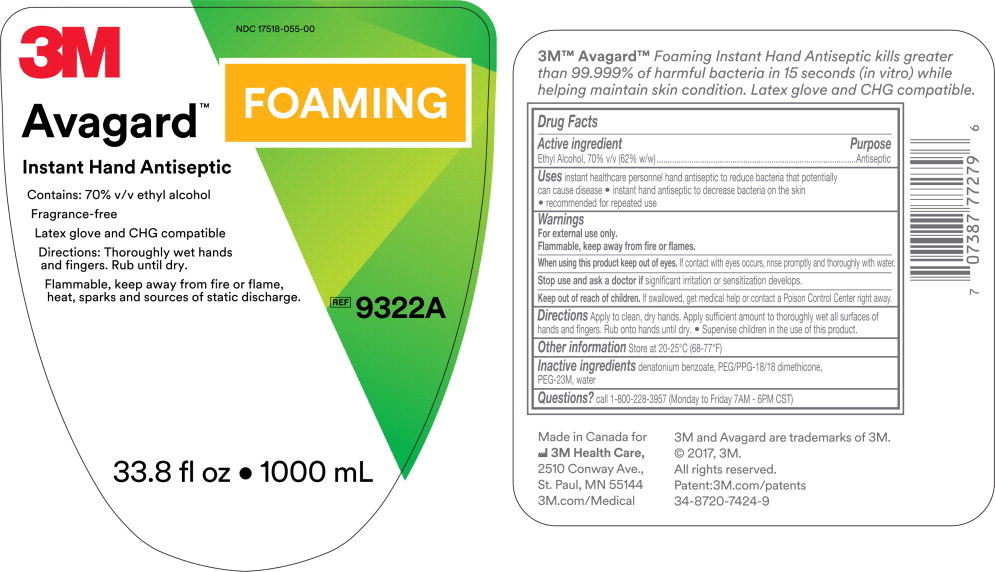
PRINCIPAL DISPLAY PANEL
3M
NDC 17518-055-02
Avagard™ Foaming
Instant Hand Antiseptic
Contains: 70% v/v ethyl alcohol
Fragrance-Free
Latex glove and CHG compatible
Flammable, keep away from fire or flame, heat, sparks and sources of static discharge.
REF
9320A
1.6 fl oz • 50 mL
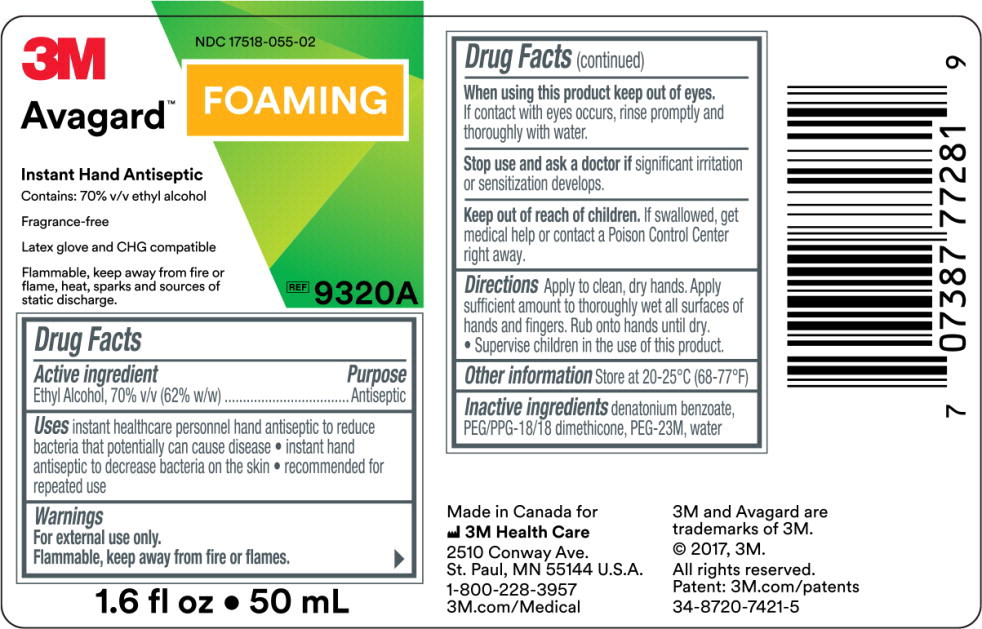
PRINCIPAL DISPLAY PANEL
3M
NDC 17518-055-01
Avagard™ Foaming
Instant Hand Antiseptic
Contains: 70% v/v ethyl alcohol
Fragrance-Free
Latex glove and CHG compatible
Flammable, keep away from fire or flame, heat, sparks and sources of static discharge.
REF
9321A
16.9 fl oz • 500 mL

PRINCIPAL DISPLAY PANEL
3M
NDC 17518-055-00
Avagard™ Foaming
Instant Hand Antiseptic
Contains: 70% v/v ethyl alcohol
Fragrance-Free
Latex glove and CHG compatible
Directions: Thoroughly wet hands and fingers. Rub until dry.
Flammable, keep away from fire or flame, heat, sparks and sources of static discharge.
REF
9322A
33.8 fl oz • 1000 mL