NDC Code(s) : 17518-060-04
Packager : Solventum US OpCo LLC
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| 3M Skin and Nasal AntisepticPovidone-Iodine SOLUTION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Solventum US OpCo LLC(006173082) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| 3M Company | 054950670 | ANALYSIS(17518-060), LABEL(17518-060), MANUFACTURE(17518-060), PACK(17518-060) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| 3M Company | 078671244 | MANUFACTURE(17518-060), ANALYSIS(17518-060) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| 3M Company | 830016148 | ANALYSIS(17518-060) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| BASF | 040776809 | API MANUFACTURE(17518-060) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Pace Analytical Life Sciences, LLC | 797903197 | ANALYSIS(17518-060) | |
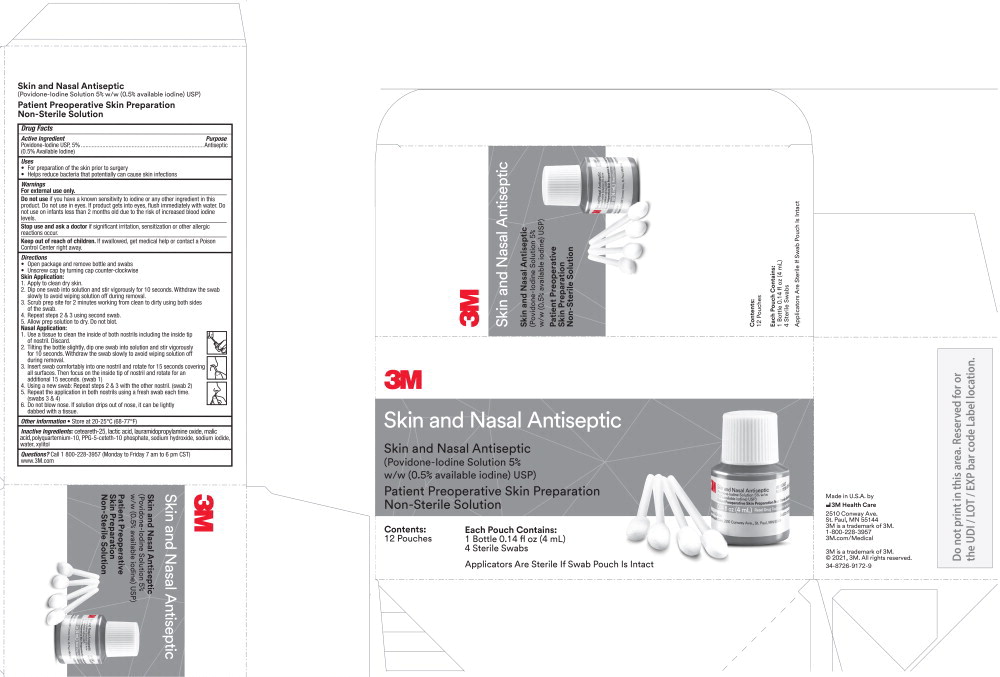
PRINCIPAL DISPLAY PANEL
3M Skin and Nasal Antiseptic
(Povidone-Iodine Solution 5% w/w (0.5% available iodine) USP)
Patient Preoperative Skin Preparation
Non-Sterile Solution
Contents:
12 pouches
Each Pouch Contains:
1 Bottle 0.14 fl oz (4 mL)
4 Sterile Swabs
Applicators Are Sterile If Swab Pouch Is Intact
Made in U.S.A. by
3M Health Care
2510 Conway Ave.
St. Paul, MN 55144
3M is a trademark of 3M
1-800-228-3957
3m.com/Medical
3M is a trademark of 3M
© 2021, 3M. All rights reserved.
34-8726-9172-9
PRINCIPAL DISPLAY PANEL
NDC 17518-060-04
Not Made With Natural Rubber Latex
Do Not Reuse
3M Skin and Nasal Antiseptic
Skin and Nasal Antiseptic
(Povidone-Iodine Solution 5% w/w (0.5% available iodine) USP)
Patient Preoperative
Skin Preparation
Non-Sterile Solution
Each Pouch Contains:
1 Bottle 0.14 fl oz (4 mL)
4 Sterile Swabs
Applicators are sterile if swab pouch is intact
REF
192401










