NDC Code(s) : 23155-170-31
Packager : Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Zoledronic acidZoledronic acid INJECTION, SOLUTION, CONCENTRATE | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc.(780779901) |
| REGISTRANT - AVET LIFESCIENCES PRIVATE LIMITED(853181664) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Emcure Pharmaceuticals Limited | 675467924 | ANALYSIS(23155-170), MANUFACTURE(23155-170), PACK(23155-170), LABEL(23155-170) | |
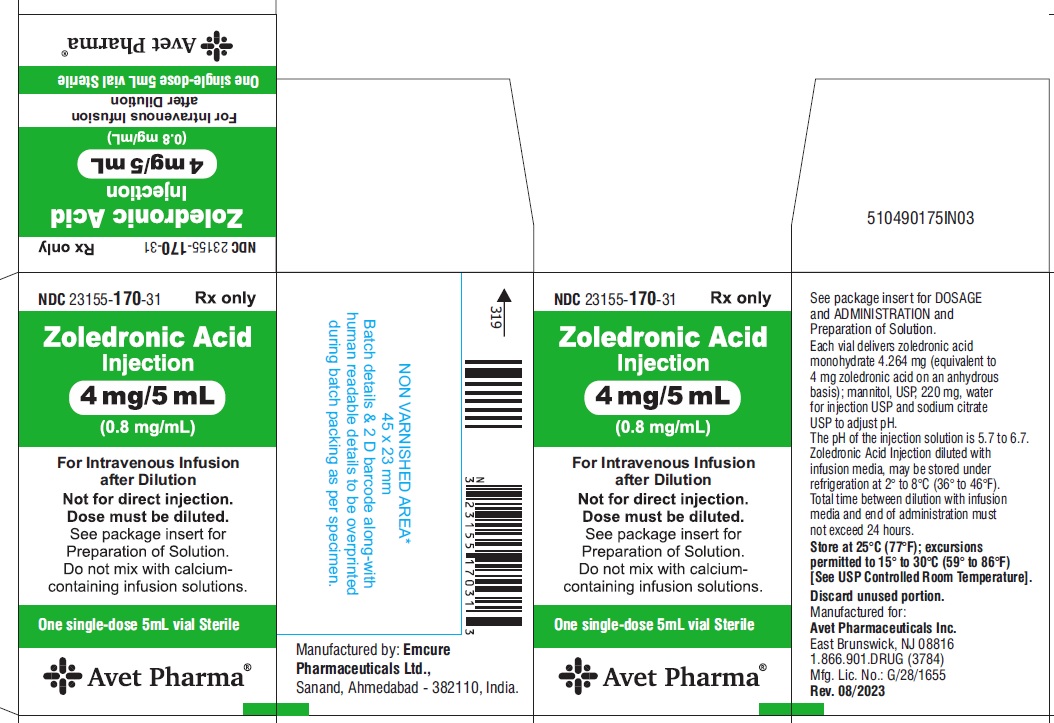
PRINCIPAL DISPLAY PANEL
NDC 23155-170-31
Zoledronic Acid Injection
4 mg/5 mL
(0.8 mg/mL)
Rx only
For Intravenous Infusion after Dilution
Not for direct injection.
Dose must be diluted.
Sterile
Single-dose 5 mL vial

PRINCIPAL DISPLAY PANEL
NDC 23155-170-31
Zoledronic Acid Injection
4 mg/5 mL
(0.8 mg/mL)
Rx only
For Intravenous Infusion after Dilution
Not for direct injection.
Dose must be diluted.
One single-dose 5 mL vial Sterile