NDC Code(s) : 23155-345-32, 23155-345-42, 23155-345-33, 23155-345-43, 23155-345-31, 23155-345-41, 23155-345-44
Packager : Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| propofolpropofol INJECTION, EMULSION | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc.(780779901) |
| REGISTRANT - AVET LIFESCIENCES PRIVATE LIMITED(853181664) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Emcure Pharmaceuticals Limited | 675467924 | ANALYSIS(23155-345), MANUFACTURE(23155-345), PACK(23155-345), LABEL(23155-345) | |
PRINCIPAL DISPLAY PANEL
NDC 23155-345-44
Propofol Injectable Emulsion, USP
200 mg/20 mL
(10 mg/mL)
For Intravenous Administration
SHAKE WELL BEFORE USING
20 x 20 mL Vials for Single Patient Use Only

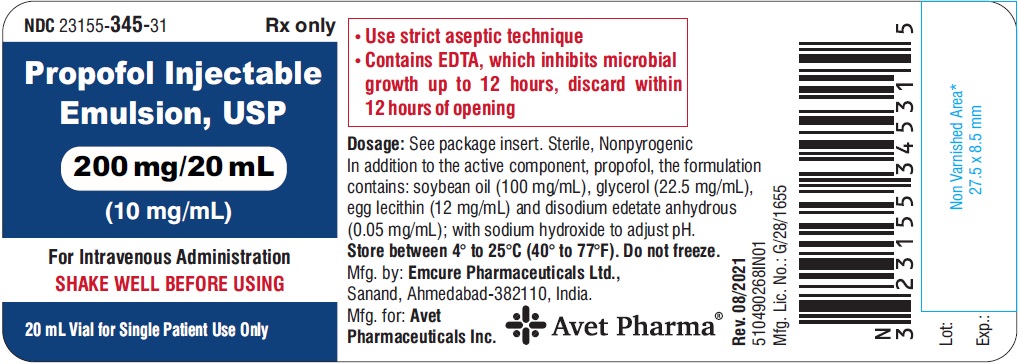
NDC 23155 - 345 - 31
Propofol Injectable Emulsion, USP
200 mg/20 mL
(10 mg/mL)
For Intravenous Administration
SHAKE WELL BEFORE USING
20 mL Vial for Single Patient Use Only
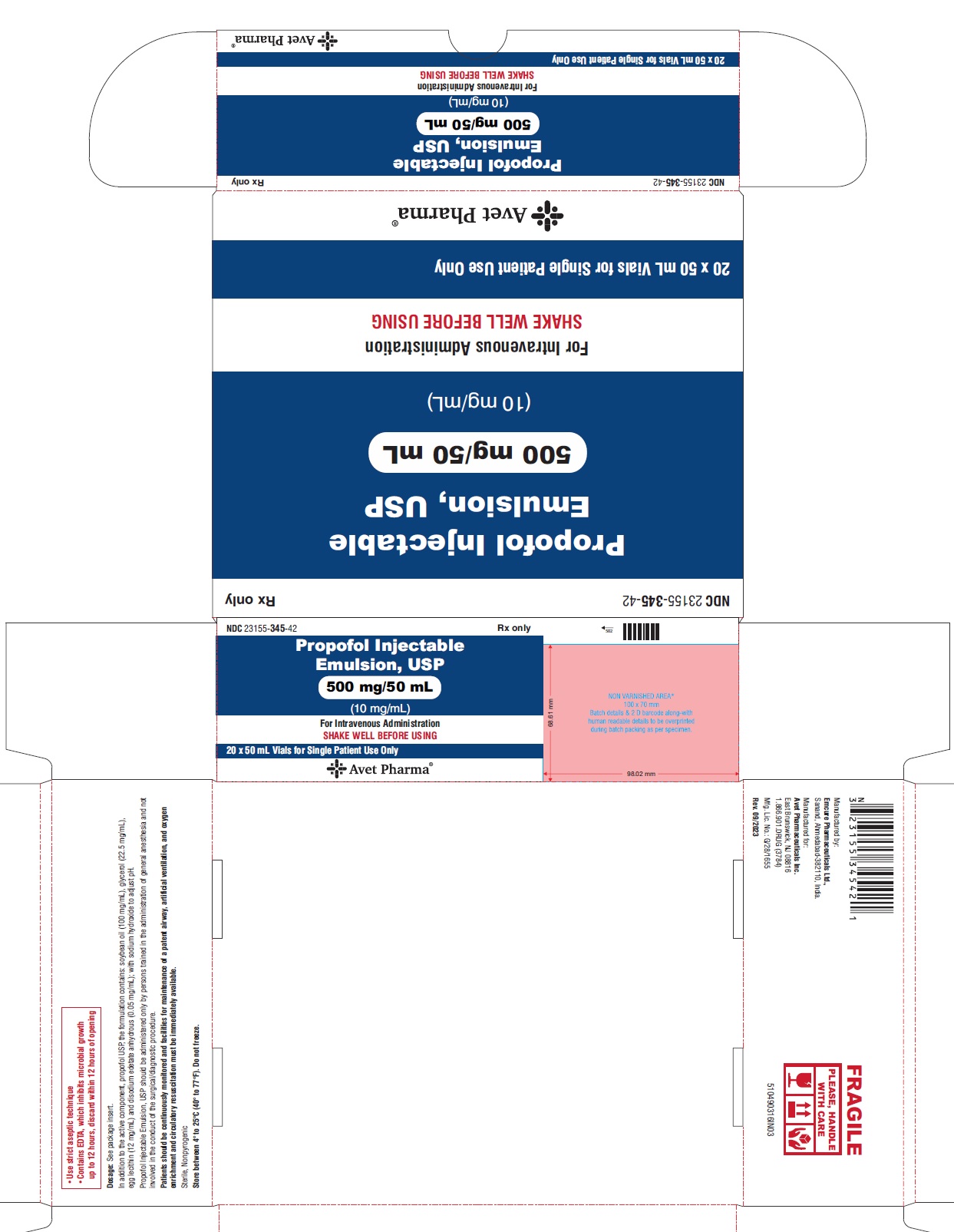
NDC 23155-345-42
Propofol Injectable Emulsion, USP
500 mg/50 mL
(10 mg/mL)
For Intravenous Administration
SHAKE WELL BEFORE USING
20 x 50 mL Vials for Single Patient Use Only
NDC 23155-345-32
Propofol Injectable Emulsion, USP
500 mg/50 mL
(10 mg/mL)
For Intravenous Administration
SHAKE WELL BEFORE USING
50 mL Vial for Single Patient Use Only

NDC 23155-345-43
Propofol Injectable Emulsion, USP
1,000 mg/100 mL
(10 mg/mL)
For Intravenous Administration
SHAKE WELL BEFORE USING
10 x 100 mL Vials for Single Patient Use Only

NDC 23155-345-33
Propofol Injectable Emulsion, USP
1,000 mg/100 mL
(10 mg/mL)
For Intravenous Administration
SHAKE WELL BEFORE USING
100 mL Vial for Single Patient Use Only










