NDC Code(s) : 24208-002-02
Packager : Bausch & Lomb Incorporated
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| VITRASEhyaluronidase, ovine INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Bausch & Lomb Incorporated(196603781) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Alliance Medical Products, Inc. (dba Siegfried Irvine) | 102688657 | ANALYSIS(24208-002), LABEL(24208-002), MANUFACTURE(24208-002), PACK(24208-002) | |
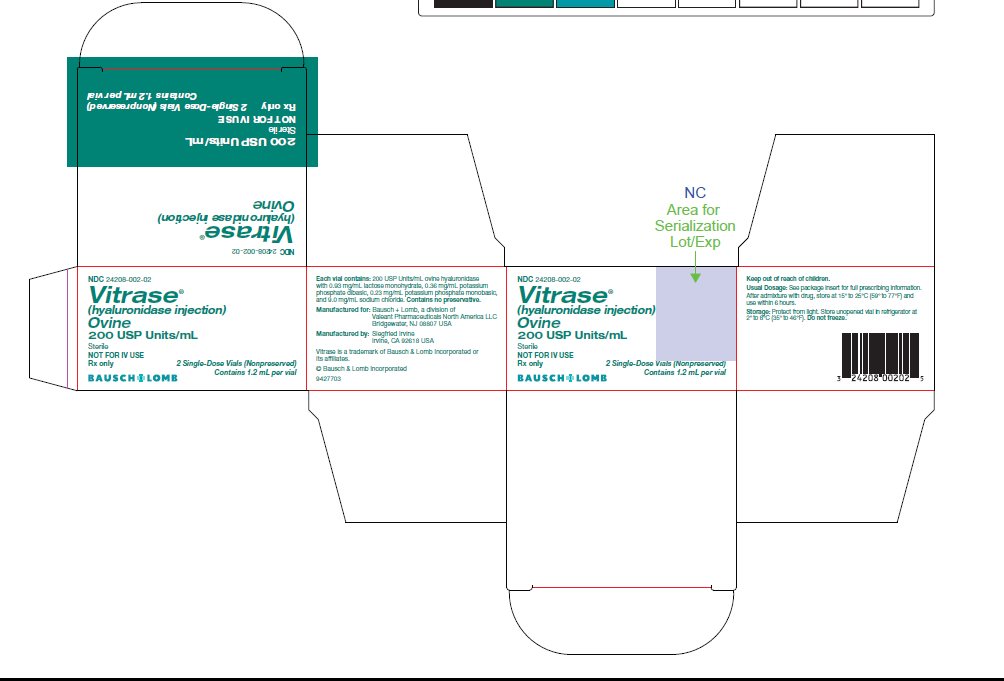
PRINCIPAL DISPLAY PANEL
NDC 24208-002-02
Vitrase®
(hyaluronidase injection)
Ovine
200 USP UNITS/mL
Sterile
NOT FOR IV USE
Rx only
2 Single-Use Vials (Nonpreserved)
Contains 1.2 mL per vial
BAUSCH+LOMB