NDC Code(s) : 24208-507-07, 24208-507-02, 24208-507-01
Packager : Bausch & Lomb Incorporated
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Lotemax SMloteprednol etabonate GEL | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LABELER - Bausch & Lomb Incorporated(196603781) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Bausch & Lomb Incorporated | 079587625 | MANUFACTURE(24208-507), PACK(24208-507), LABEL(24208-507) | |
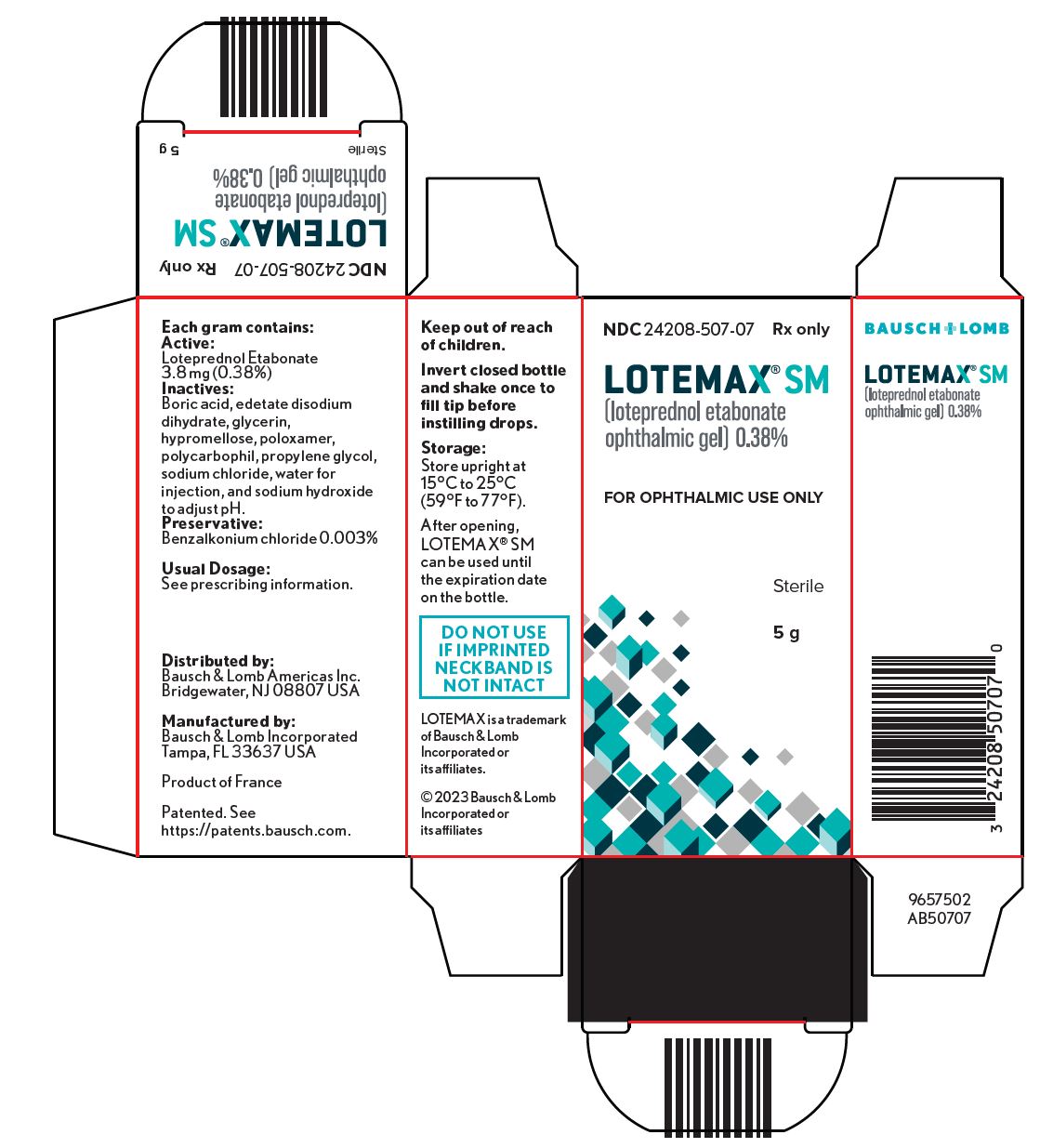
PRINCIPAL DISPLAY PANEL
NDC 24208-507-07
Rx only
Lotemax® SM
(loteprednol etabonate
Ophthalmic gel) 0.38%
FOR OPHTHALMIC USE ONLY
Sterile
5 g