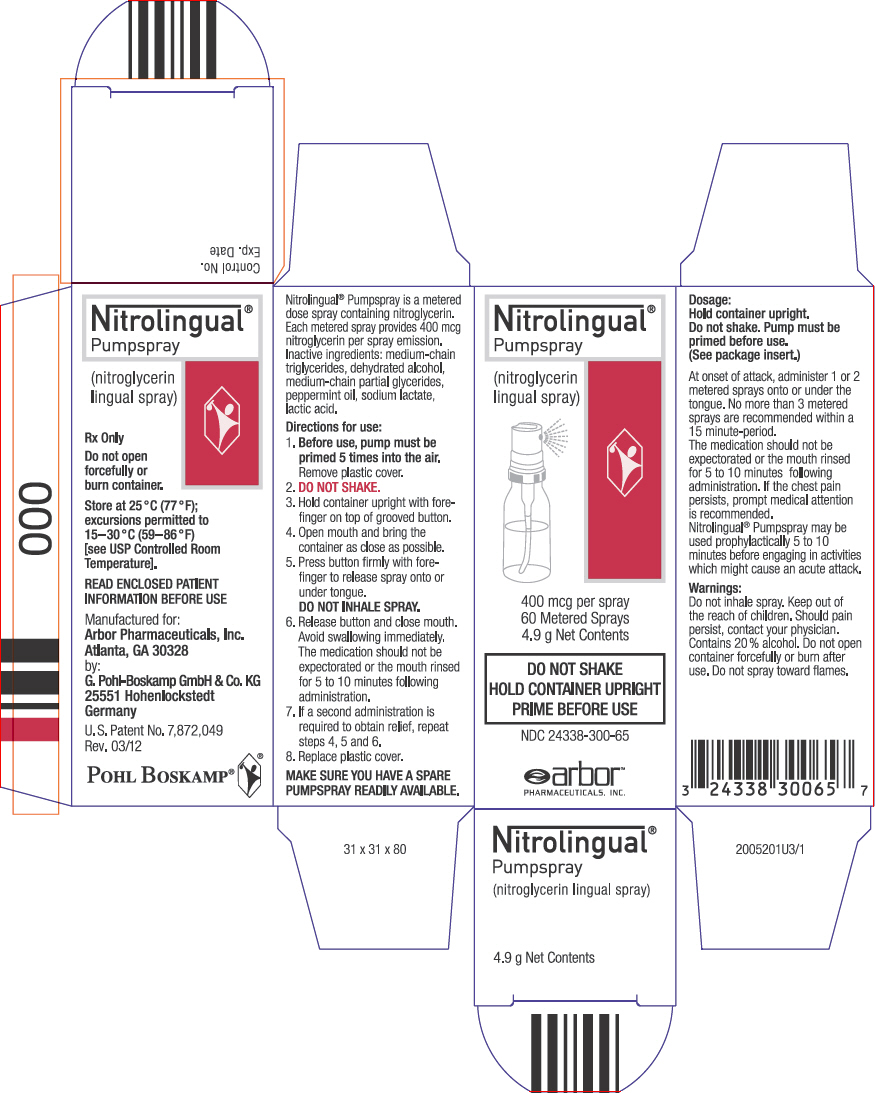
NDC Code(s) : 24338-300-65, 24338-300-66, 24338-300-20
Packager : Arbor Pharmaceuticals, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Nitrolingual Pumpspraynitroglycerin SPRAY, METERED | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Nitrolingual
®
Pumpspray
(nitroglycerin
lingual spray)
400 mcg per spray
60 Metered Sprays
4.9 g Net Contents
DO NOT SHAKE
HOLD CONTAINER UPRIGHT
PRIME BEFORE USE
NDC 24338-300-65
arbor™
PHARMACEUTICALS, INC.