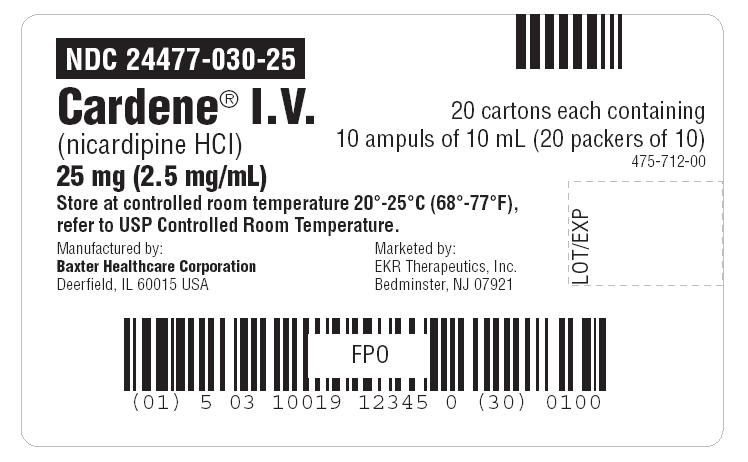
NDC Code(s) : 24477-030-10, 24477-030-25
Packager : EKR Therapeutics
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Cardene I.V.nicardipine hydrochloride INJECTION | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Package Label - Principal Display Panel – Vial label

PRINCIPAL DISPLAY PANEL
Container Label - Principal Display Panel – Vial's single box label

PRINCIPAL DISPLAY PANEL
Container Label - Principal Display Panel – 10pck carton label

PRINCIPAL DISPLAY PANEL
Container Label - Principal Display Panel – 20crtn Case label