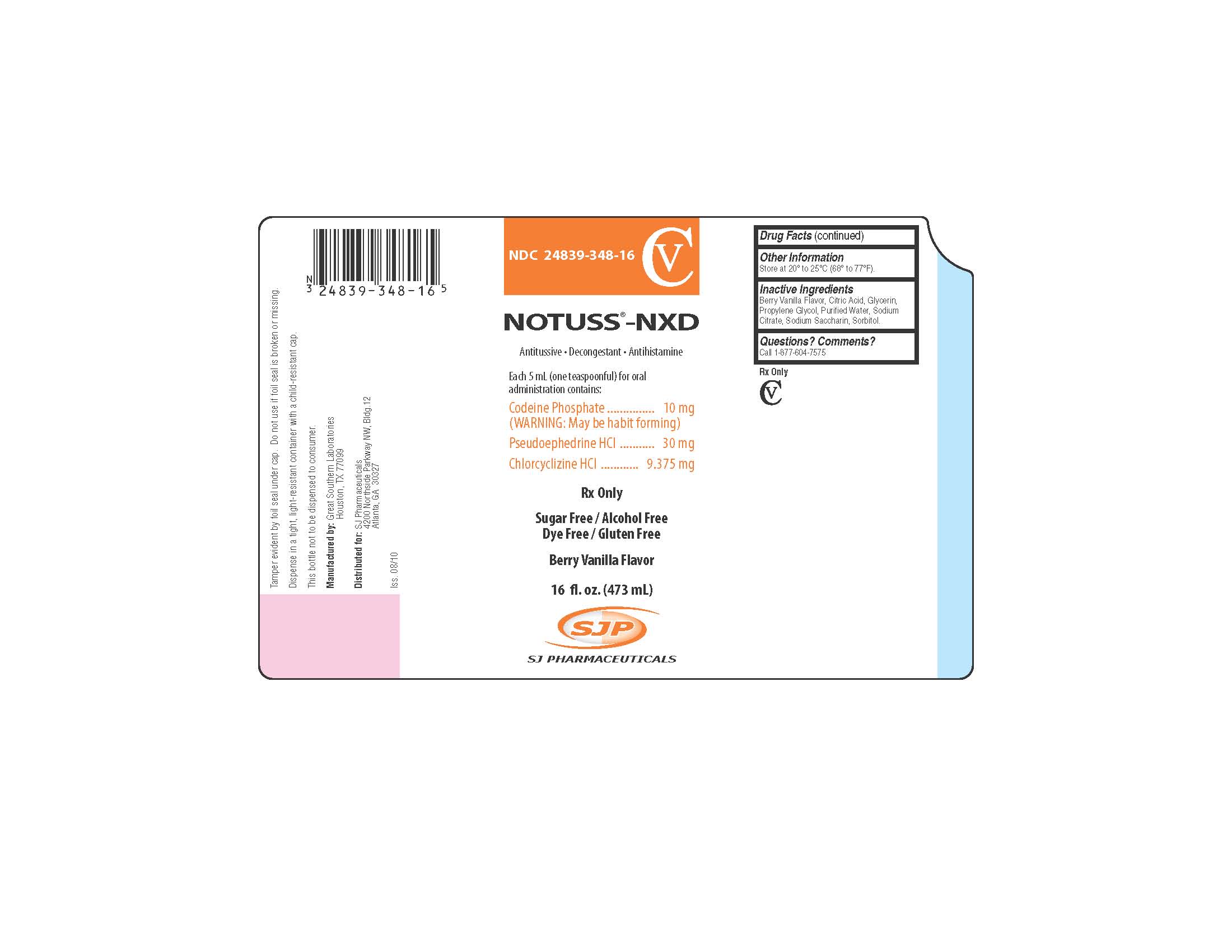
NDC Code(s) : 24839-348-16, 24839-348-10
Packager : SJ PHARMACEUTICALS, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Notuss-NXD Codeine Phosphate, Pseudoephedrine HCl, Chlorcyclizine HCl LIQUID | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
NOTUSS®-NXD
Antitussive - Decongestant - Antihistamine
Each 5 mL (one teaspoonful) for oral administration contains:Codeine Phosphate .................................................. 10 mg
(WARNING: May be habit forming)
Pseudoephedrine HCl .............................................. 30 mg
Chlorcyclizine HCl ............................................... 9.375 mg
Rx Only
Sugar Free/Alcohol Free
Dye Free/Gluten Free
16 fl. oz. (473 mL)
Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
Dispense in a tight, light-resistant container with a child-resistant cap.
This bottle not to be dispensed to consumer.
Manufactured by:
Great Southern Laboratories
Houston, TX 77099
SJ Pharmaceuticals
4200 Northside Parkway NW, Bldg. 12
Atlanta, GA 30327
Iss. 08/10