NDC Code(s) : 24987-562-10, 24987-562-20
Packager : Covis Pharmaceuticals Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| PlaquenilHydroxychloroquine Sulfate TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
NDC 24987-562-10
Rx only
Plaquenil®
hydroxychloroquine
sulfate, USP
200 mg
100 tablets
KEEP OUT OF THE REACH OF CHILDREN
Dispense in a tight, light-resistant container
as defined in the USP/NF.
COVIS
Mfd. for: Covis Pharmaceuticals, Inc.
Cary, NC 27511
©2013 Covis Pharmaceuticals, Inc. 50106153
Origin France Rev. 5/13 100117
Store at room temperature up to 30° C (86°F).
For indications and dosage, see package insert.
PHARMACIST: Children are especially sensitive to this medication. Tablets should be kept out of their reach.
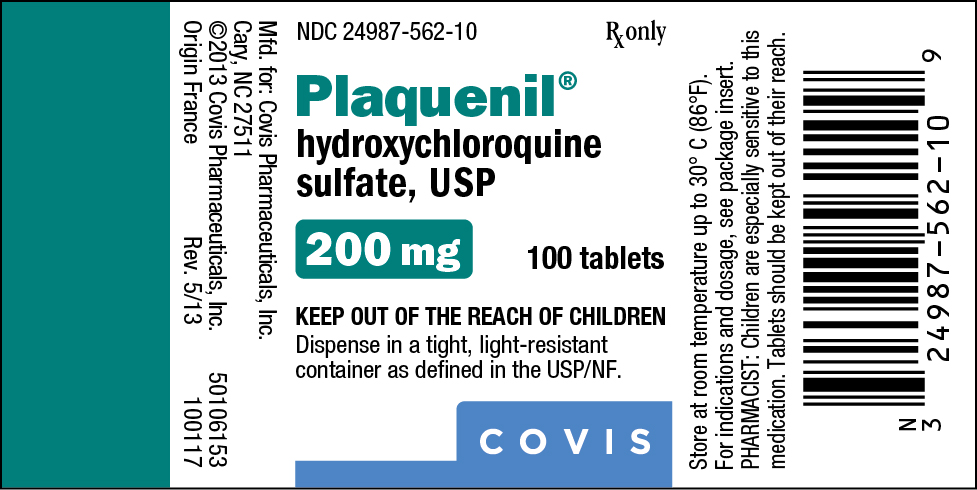 Bottle Label
Bottle Label
PRINCIPAL DISPLAY PANEL
GOVERNMENT USE ONLY
NDC 24987-562-20
Rx only
Plaquenil®
hydroxychloroquine
sulfate, USP
200 mg
100 tablets
KEEP OUT OF THE REACH OF CHILDREN
Dispense in a tight, light-resistant container
as defined in the USP/NF.
COVIS
Mfd. for: Covis Pharmaceuticals, Inc.
Cary, NC 27511
©2013 Covis Pharmaceuticals, Inc. 50106158
Origin France Rev. 5/13 100119
Store at room temperature up to 30° C (86°F).
For indications and dosage, see package insert.
PHARMACIST: Children are especially sensitive to this medication. Tablets should be kept out of their reach.
 Bottle Label
Bottle Label









