NDC Code(s) : 25010-816-56, 25010-817-56
Packager : Aton Pharma, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Timolol MaleateTimolol Maleate SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Timolol MaleateTimolol Maleate SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
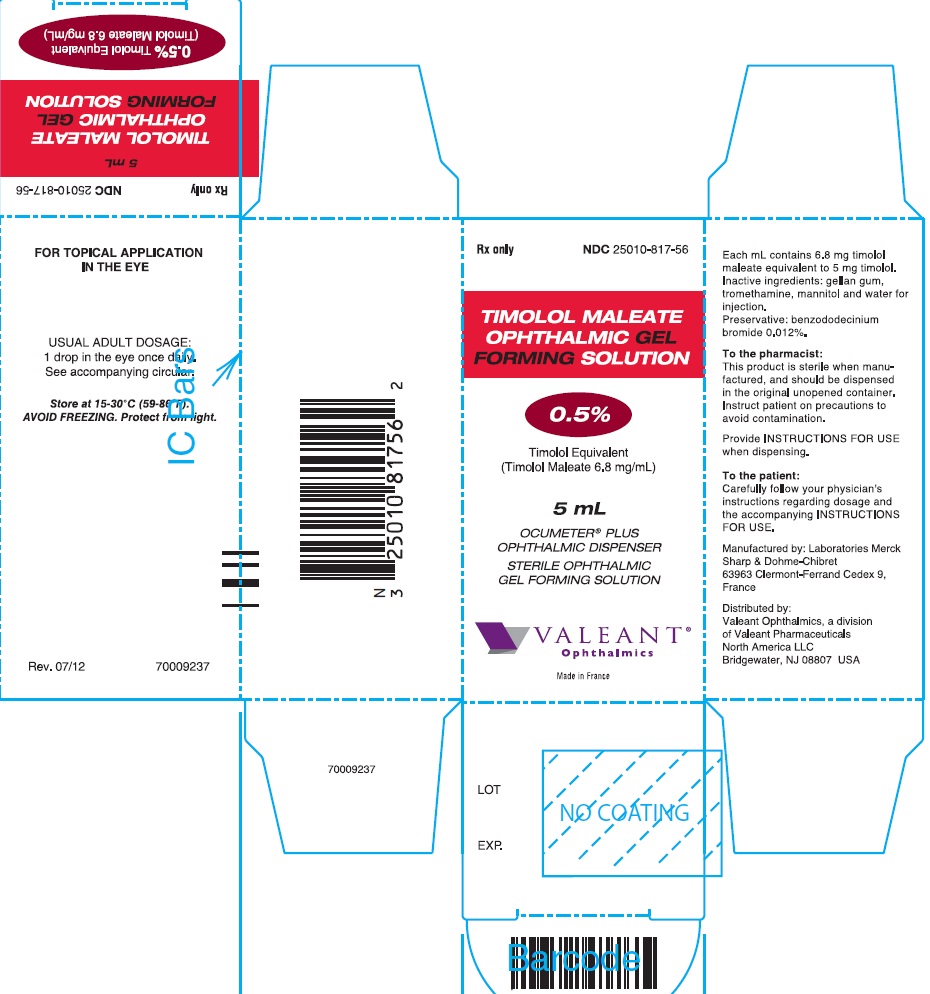
PRINCIPAL DISPLAY PANEL
Rx only
NDC 25010-816-56
TIMOLOL MALEATE
OPHTHALMIC GEL
FORMING SOLUTION
0.25%
Timolol Equivalent
(Timolol Maleate 3.4 mg/mL)
5 mL
OCUMETER® PLUS
OPHTHALMIC DISPENSER
STERILE OPHTHALMIC
GEL FORMING SOLUTION
VALEANT®
Ophthalmics
Made in France
Rev. 07/12
70009234
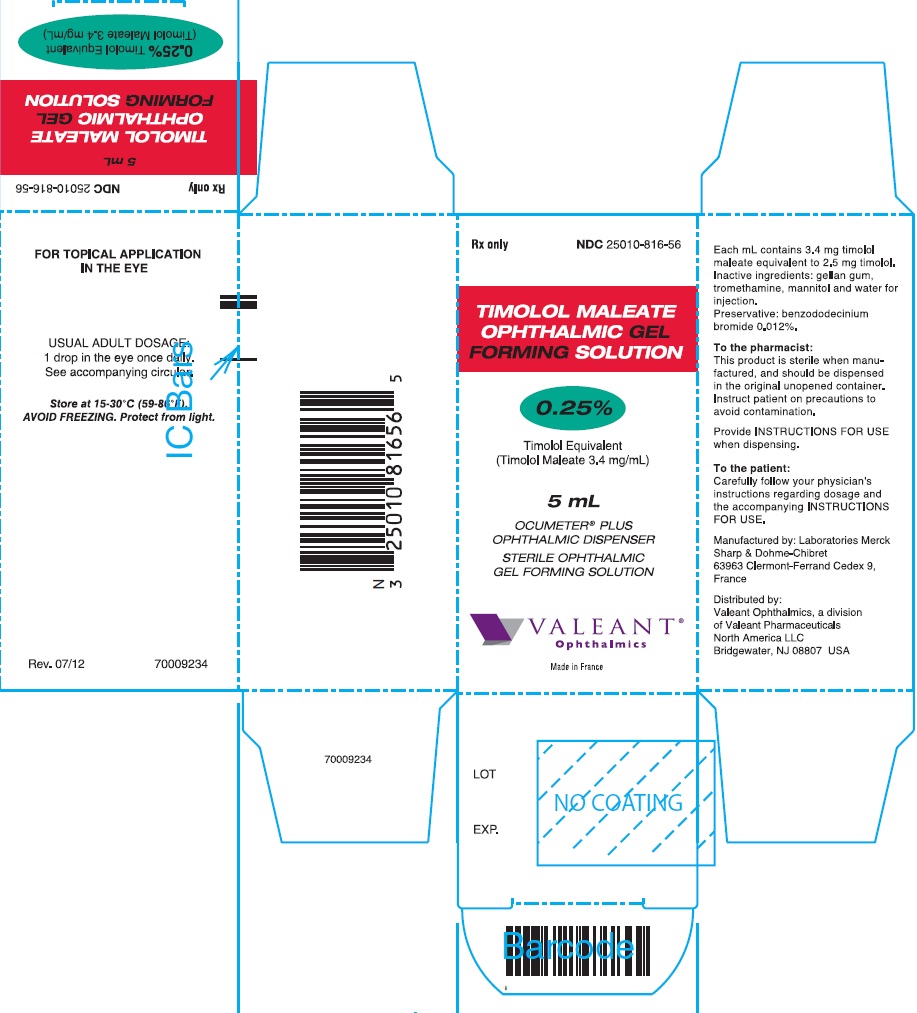
PRINCIPAL DISPLAY PANEL
Rx only
NDC 25010-817-56
TIMOLOL MALEATE
OPHTHALMIC GEL
FORMING SOLUTION
0.5%
Timolol Equivalent
(Timolol Maleate 6.8 mg/mL)
5 mL
OCUMETER® PLUS
OPHTHALMIC DISPENSER
STERILE OPHTHALMIC
GEL FORMING SOLUTION
VALEANT®
Ophthalmics
Made in France
Rev. 07/12
70009237