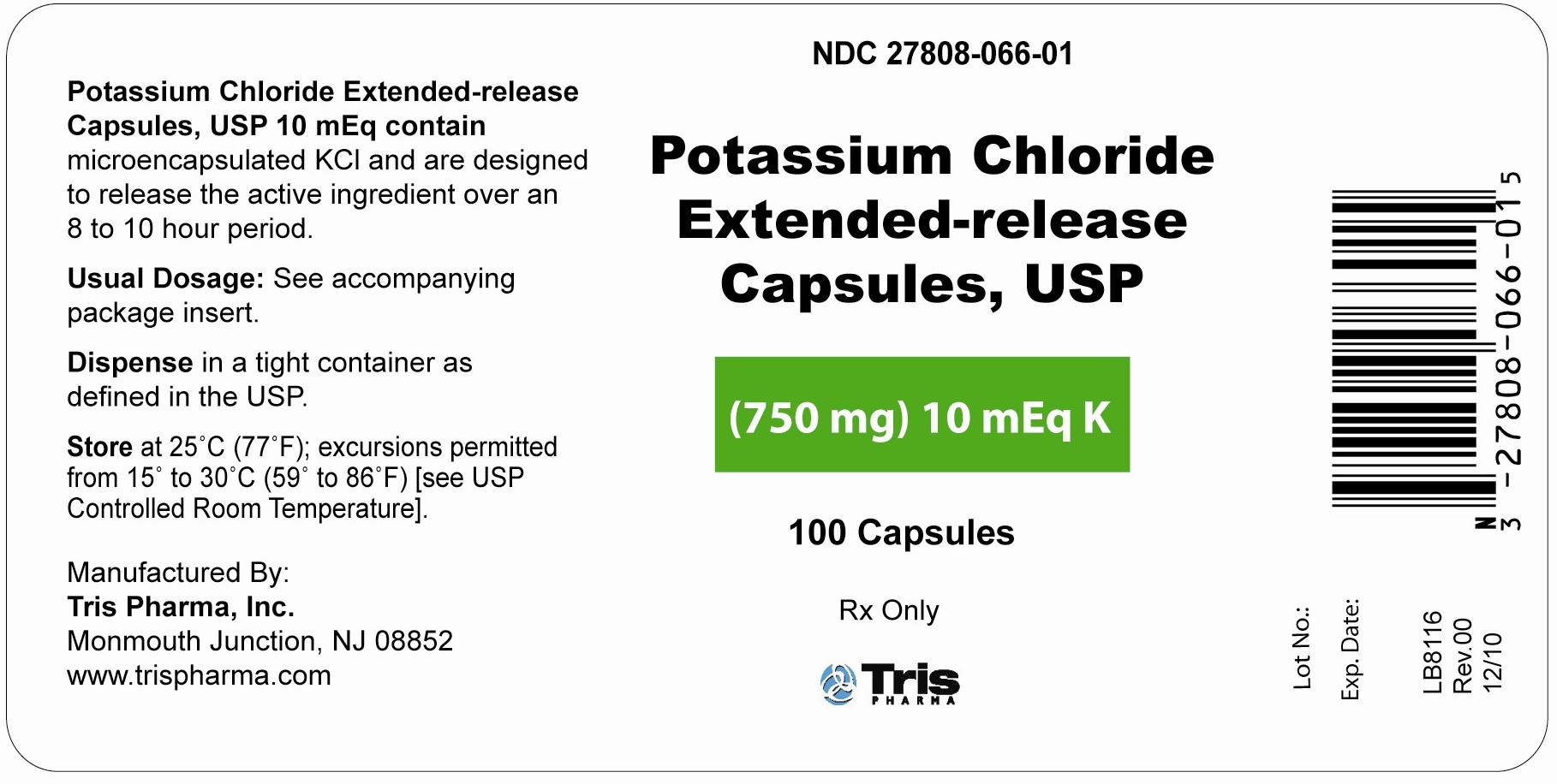
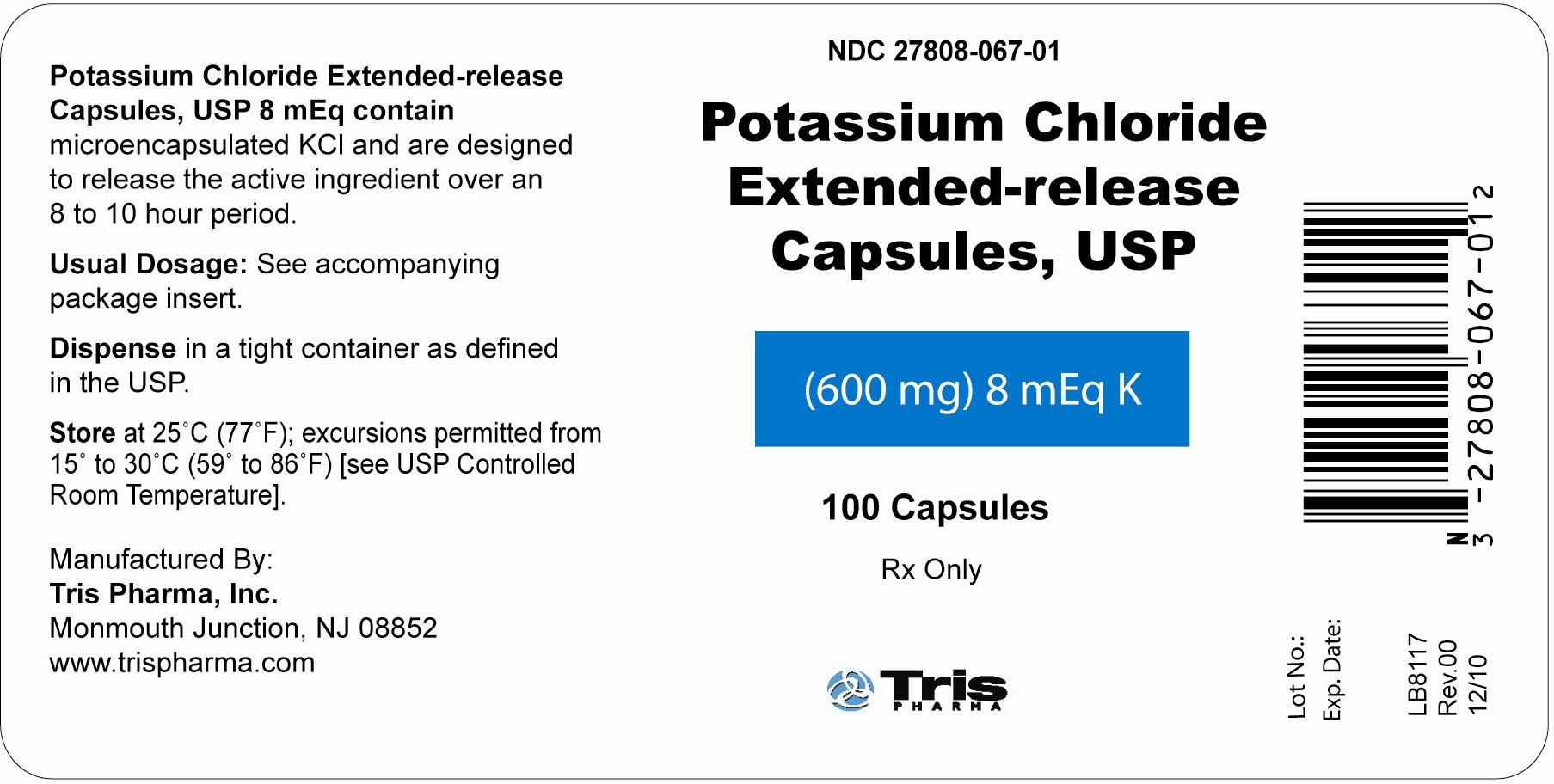
NDC Code(s) : 27808-067-01, 27808-066-01
Packager : Tris Pharma Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Potassium ChloridePotassium Chloride CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Potassium ChloridePotassium Chloride CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Label (600 mg) 8 mEq K Bottles of 100
Label (750 mg) 10 mEq K Bottles of 100