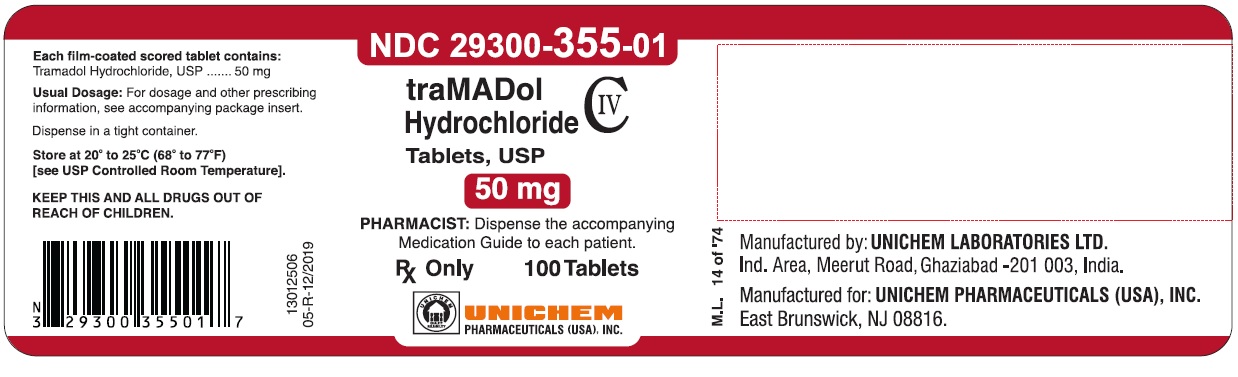
NDC Code(s) : 29300-355-01, 29300-355-05, 29300-355-10
Packager : Unichem Pharmaceuticals (USA), Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CIV
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Tramadol HydrochlorideTramadol Hydrochloride TABLET, FILM COATED | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Unichem Pharmaceuticals (USA), Inc.(181620514) |
| REGISTRANT - Unichem Laboratories Limited, India(650055882) |
PRINCIPAL DISPLAY PANEL