NDC Code(s) : 30142-121-40
Packager : The Kroger Co
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| IbuprofenIbuprofen CAPSULE, LIQUID FILLED | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Kroger®
Ibuprofen Capsules 200 mg
Pain Reliever/ Fever Reducer (NSAID)
Soft gelatin capsules**
**(liquid filled capsules)
Compare to the active ingredient in ADVIL® Liqui- Gels® †see bottom panel
Important: Read all product information before using
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
DO NOT USE IF SEAL UNDER BOTTLE CAP IMPRINTED WITH "SEALED FOR YOUR PROTECTION" IS BROKEN OR MISSING
Distributed by The Kroger Co.
Cincinnati Ohio 45202
†Advil†® is a registered trademark of pfizer consumer healthcare, Madison, NJ 07940. Pfizer consumer healthcare is not affiliated with The Kroger Co. or this product
PRINCIPAL DISPLAY PANEL
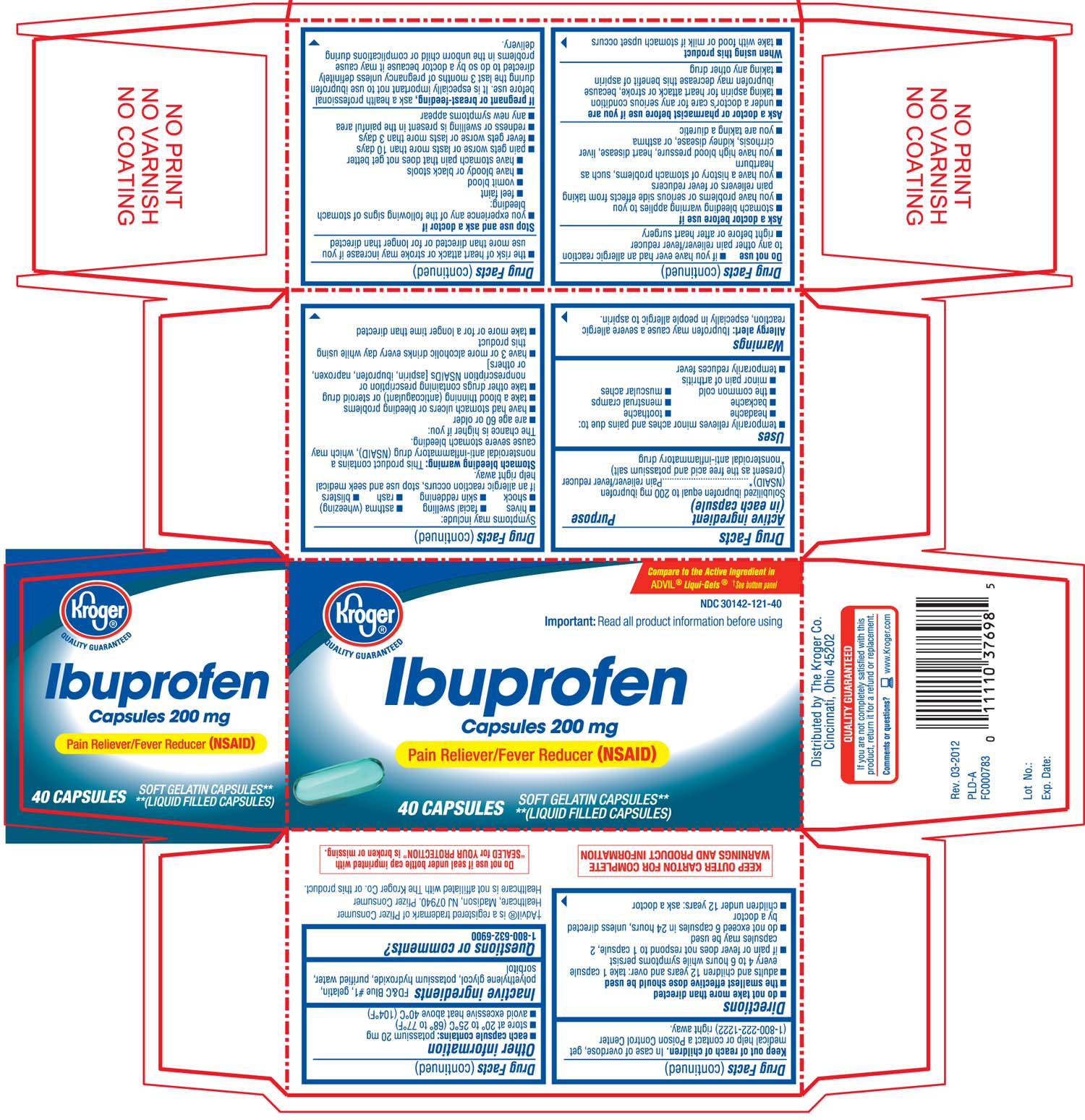 Ibuprofen Capsules 200 mg
Ibuprofen Capsules 200 mg









