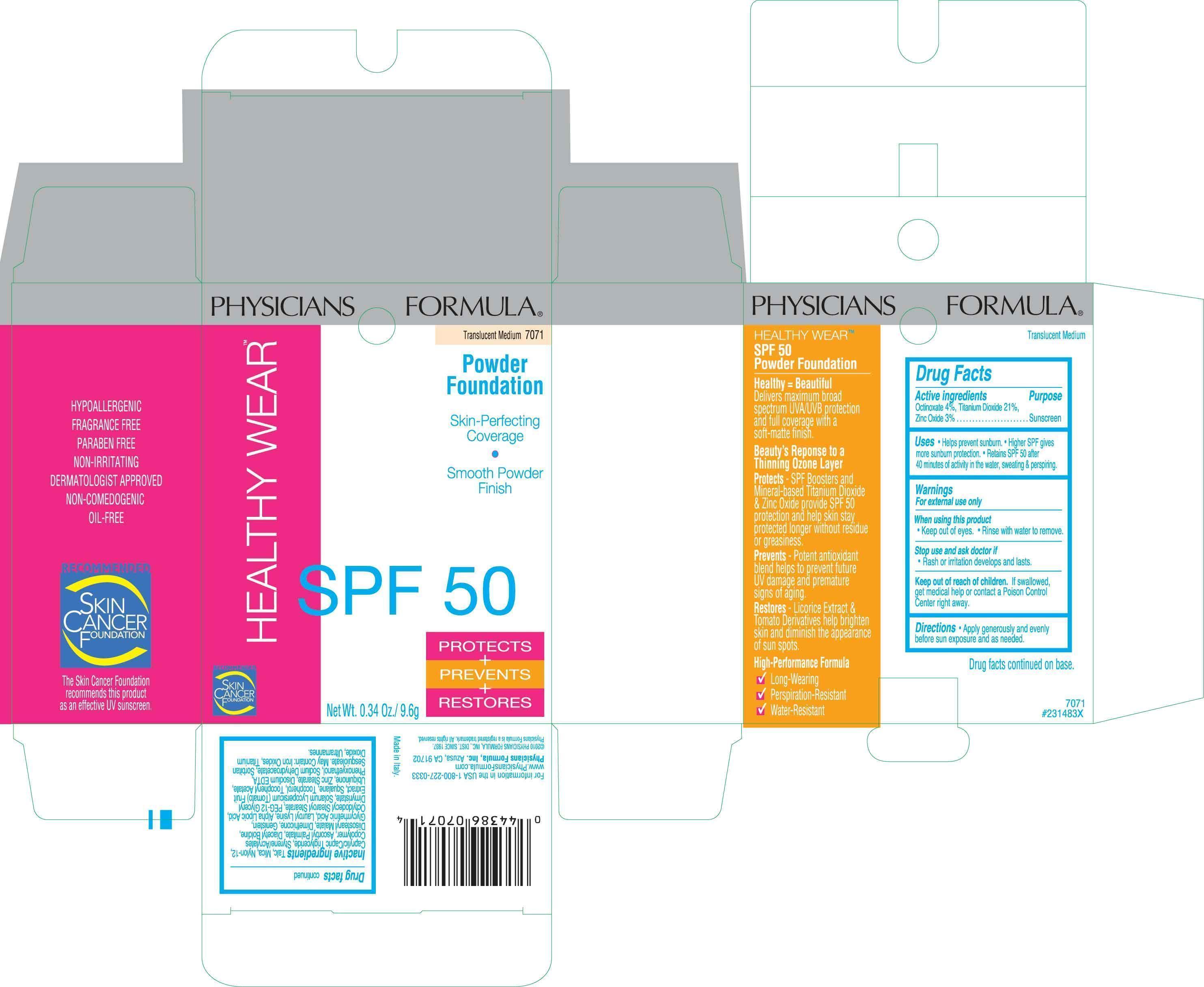
NDC Code(s) : 31645-151-01, 31645-151-02
Packager : Physicians Formula Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Healthy Wear Powder Foundation Octinoxate, Titanium Dioxide, Zinc Oxide POWDER | ||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL