NDC Code(s) : 31720-710-17
Packager : S+
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Bright future skin tint SPF 25 44 PralineOCTINOXATE, TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - S+(572406531) |
PRINCIPAL DISPLAY PANEL
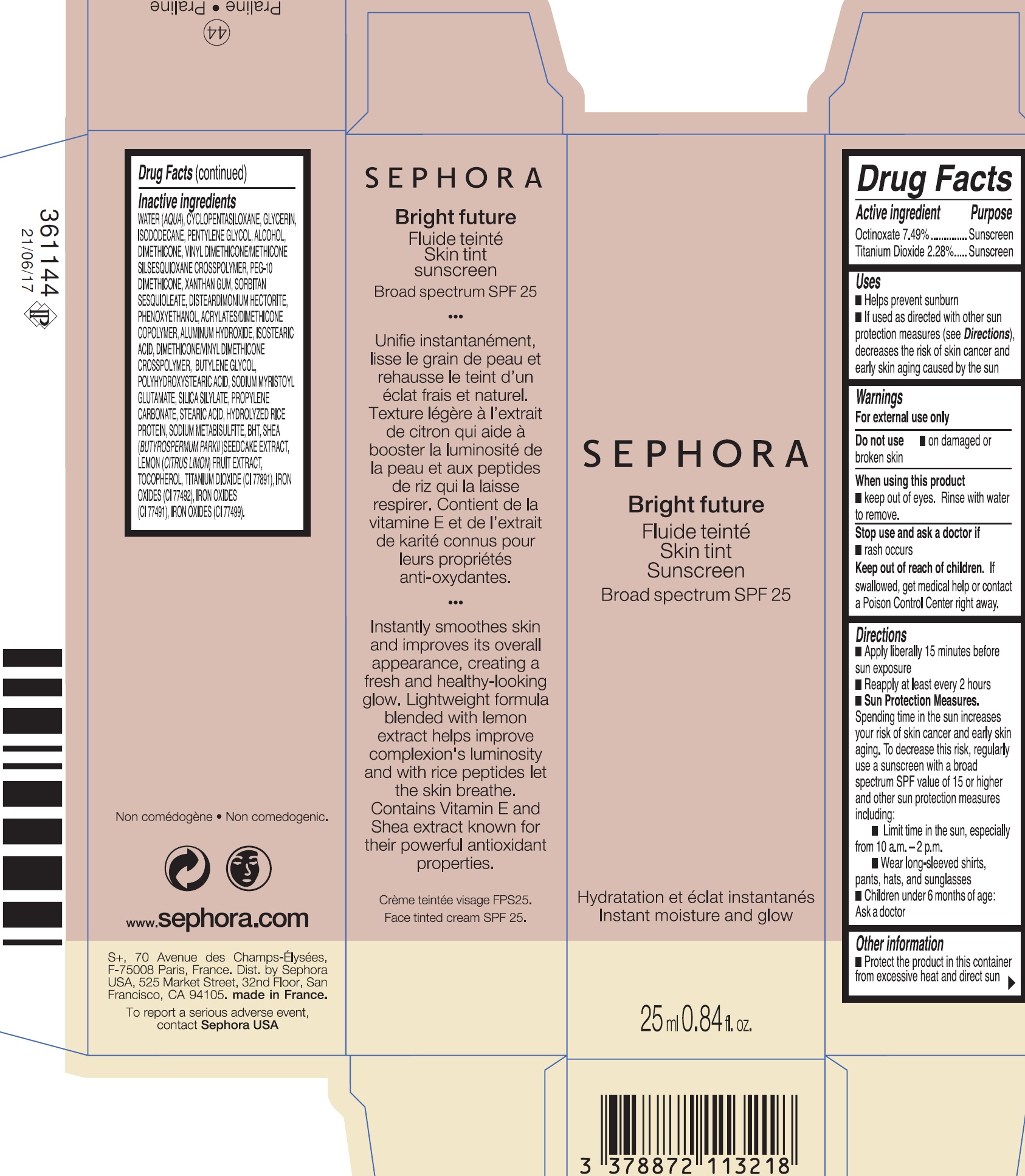
PRINCIPAL DISPLAY PANEL










