NDC Code(s) : 33342-060-07, 33342-060-15, 33342-060-12, 33342-060-10, 33342-060-44
Packager : Macleods Pharmaceuticals Limited
Category : Human Prescription Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Clopidogrel Clopidogrel TABLET | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| LABELER - Macleods Pharmaceuticals Limited(862128535) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Macleods Pharmaceuticals Limited | 676369519 | ANALYSIS(33342-060), LABEL(33342-060), MANUFACTURE(33342-060), PACK(33342-060) | |
PRINCIPAL DISPLAY PANEL
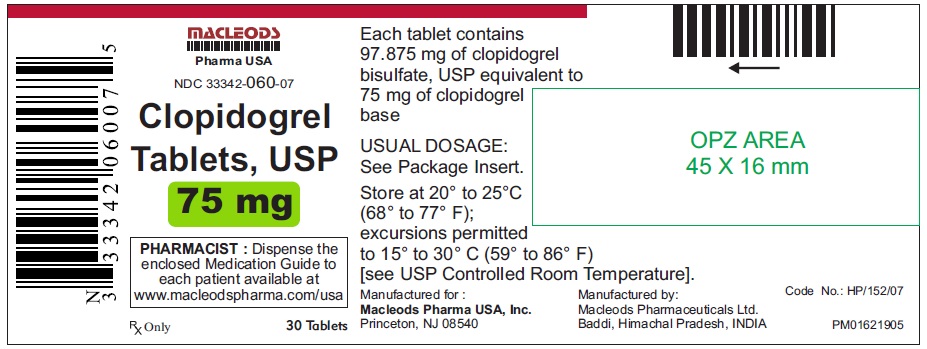
30 Tablets Container.
NDC 33342-060-07
Clopidogrel Tablets USP
Rx only
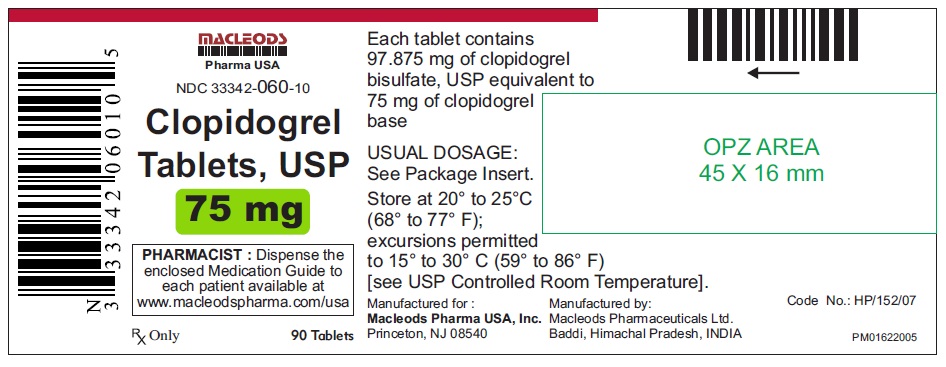
90 Tablets Container.
NDC 33342-060-10
Clopidogrel Tablets USP
Rx only
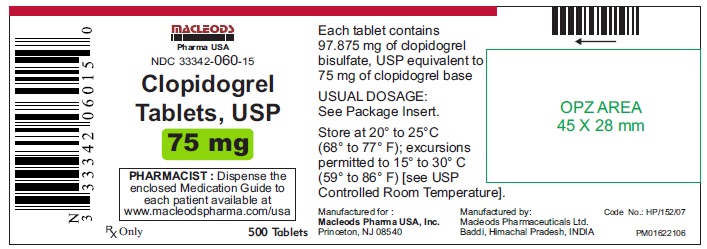
500 Tablets Container.
NDC 33342-060-15
Clopidogrel Tablets USP
Rx only
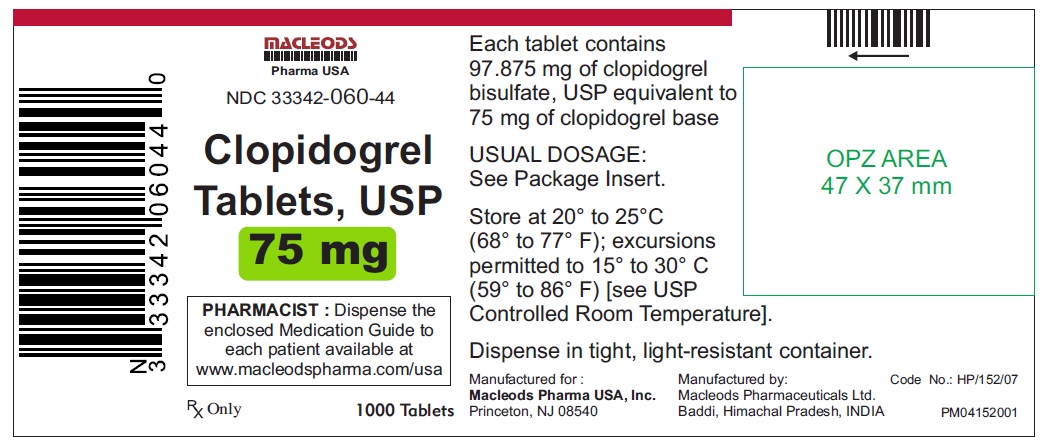
1000 Tablets Container.
NDC 33342-060-44
Clopidogrel Tablets USP
Rx only
Blister pack of 100 tablets (10 x 10 Unit Dose).
NDC 33342-060-12
Clopidogrel Tablets USP
Rx only










