NDC Code(s) : 35000-024-60, 35000-024-34, 35000-024-33, 35000-024-21, 35000-024-78
Packager : Colgate-Palmolive Company
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Colgate Sensitive Complete ProtectionPOTASSIUM NITRATE and SODIUM FLUORIDE PASTE, DENTIFRICE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Colgate-Palmolive Company(001344381) |
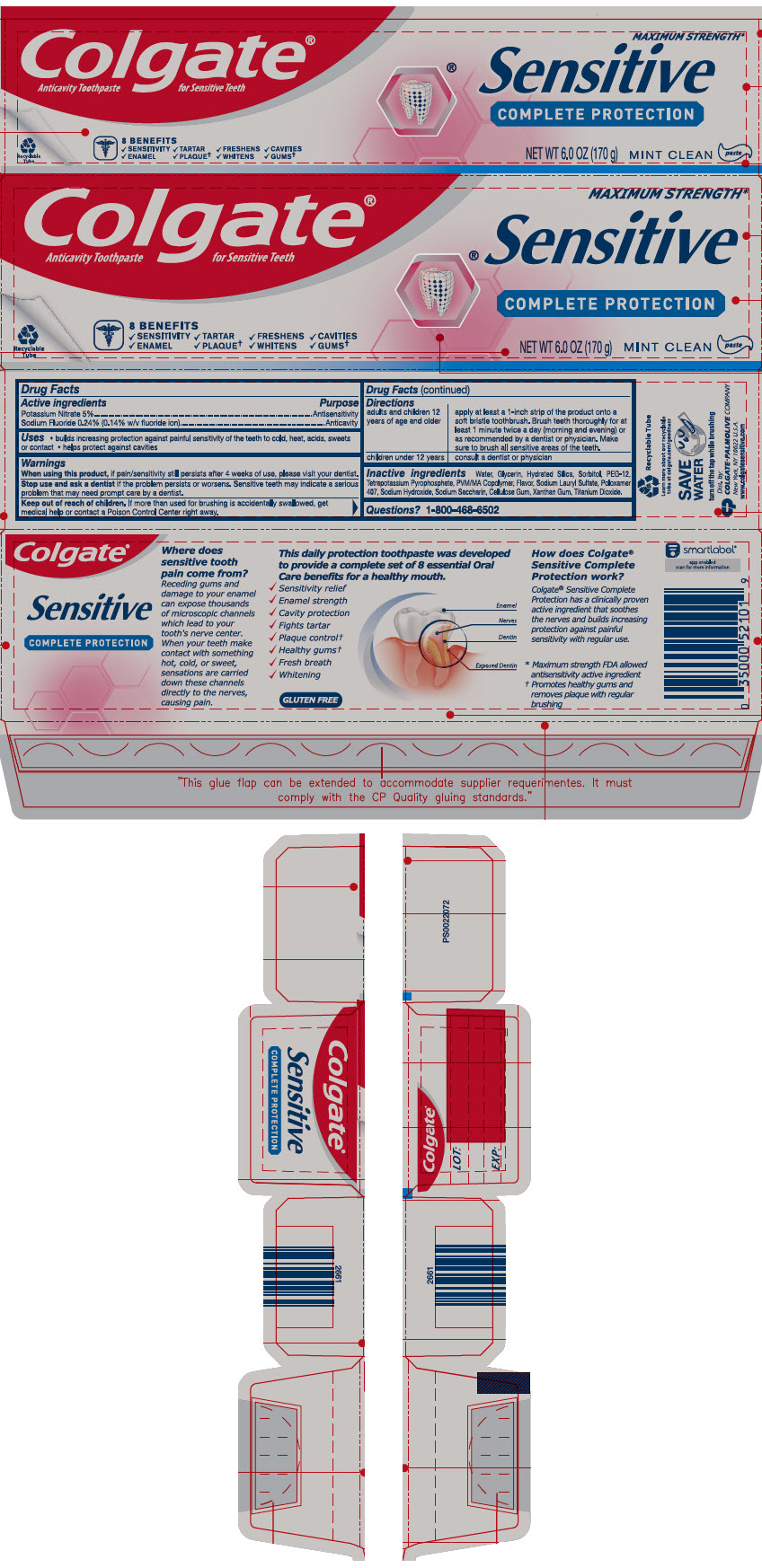
PRINCIPAL DISPLAY PANEL
Colgate®
Anticavity Toothpaste for Sensitive Teeth
MAXIMUM STRENGTH*
Sensitive
COMPLETE PROTECTION
Recyclable
Tube
8 BENEFITS
✔SENSITIVITY ✔TARTAR ✔FRESHENS ✔CAVITIES
✔ENAMEL ✔PLAQUE† ✔WHITENS ✔GUMS†
NET WT 6.0 OZ (170 g)
MINT CLEAN
paste