NDC Code(s) : 35356-118-30, 35356-333-30, 35356-339-30, 35356-339-00
Packager : Lake Erie Medical DBA Quality Care Products LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| AVINZA morphine sulfate CAPSULE, COATED, EXTENDED RELEASE | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| AVINZA morphine sulfate CAPSULE, EXTENDED RELEASE | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| AVINZA morphine sulfate CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
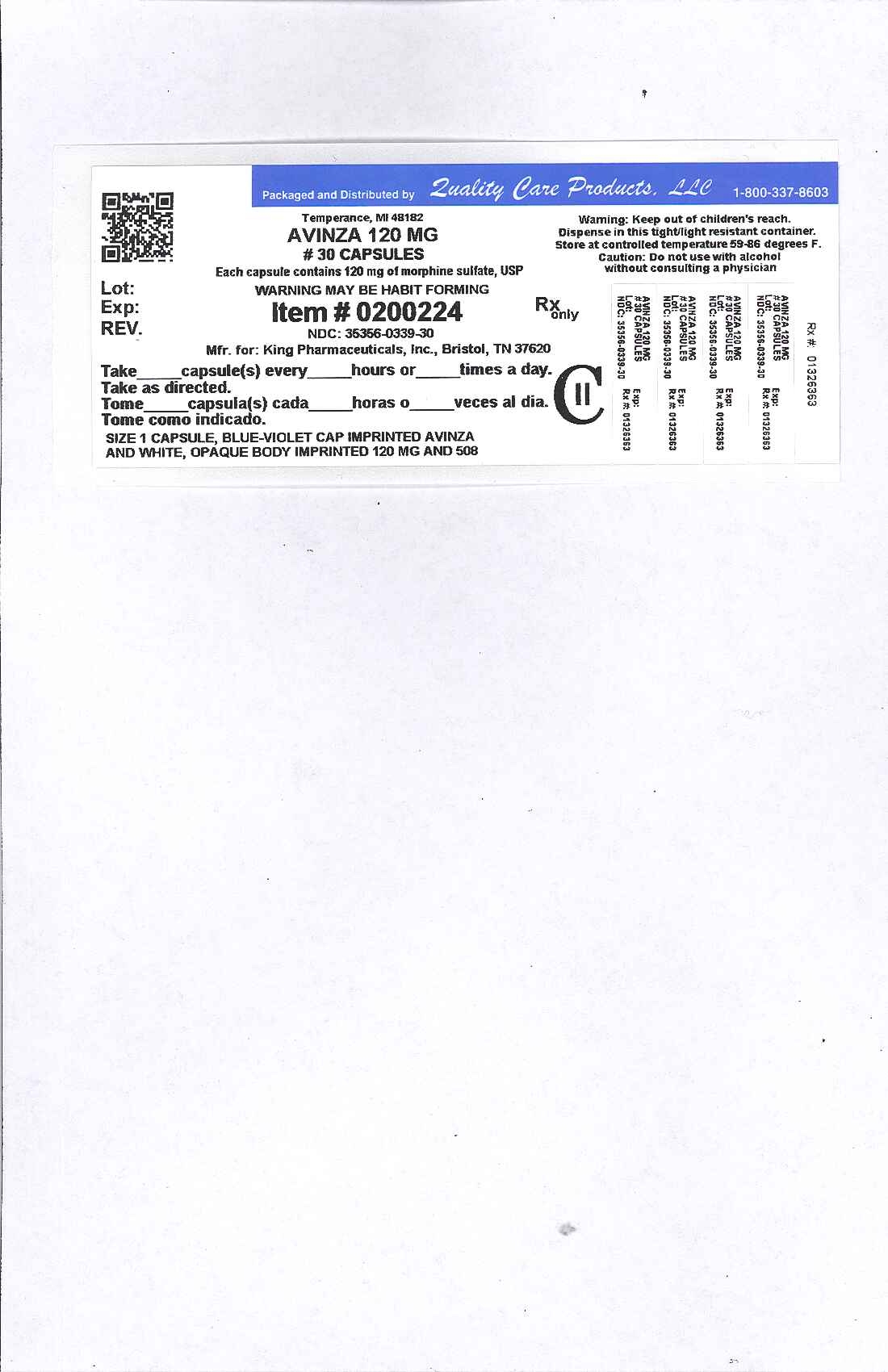
PRINCIPAL DISPLAY PANEL
image of label
PRINCIPAL DISPLAY PANEL
image of label
PRINCIPAL DISPLAY PANEL
image of label