NDC Code(s) : 37000-873-04, 37000-873-08, 37000-873-12, 37000-873-85, 37000-873-36, 37000-873-39, 37000-873-27, 37000-873-52, 37000-873-24, 37000-873-35, 37000-873-93, 37000-873-78
Packager : The Procter & Gamble Manufacturing Company
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Crest 3D White sodium fluoride PASTE, DENTIFRICE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - The Procter & Gamble Manufacturing Company(004238200) |
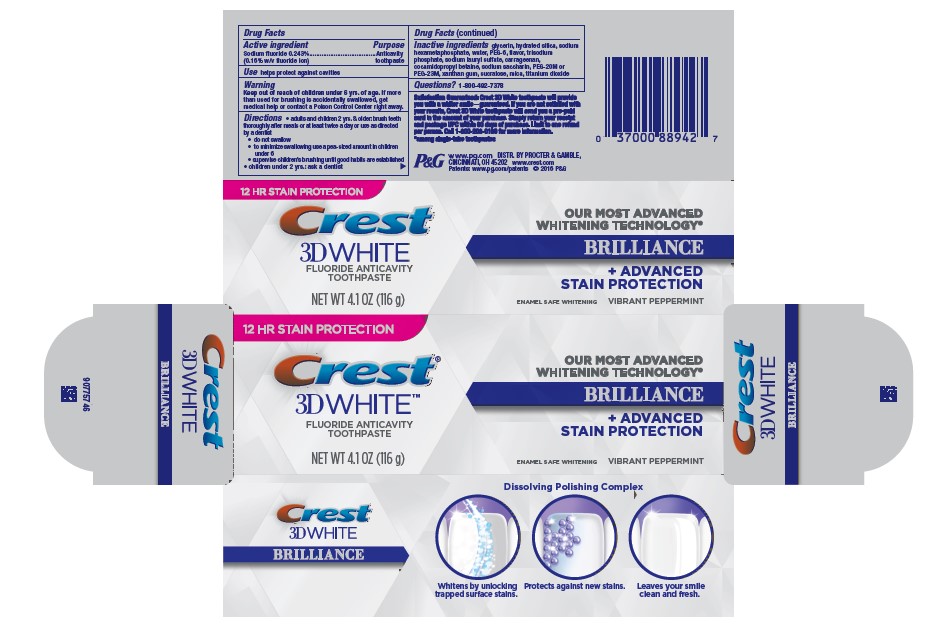
PRINCIPAL DISPLAY PANEL
12 HR STAIN PROTECTION
Crest
®
3D WHITE™
FLUORIDE ANTICAVITYTOOTHPASTE
NET WT 4.1 OZ (116 g)
OUR MOST ADVANCED
WHITENING TECHNOLOGY*
BRILLIANCE
+ ADVANCED STAIN PROTECTION
ENAMEL SAFE WHITENING VIBRANT PEPPERMINT