NDC Code(s) : 37205-203-71, 37205-203-46
Packager : Cardinal Health
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Leader NicotineNicotine GUM, CHEWING | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
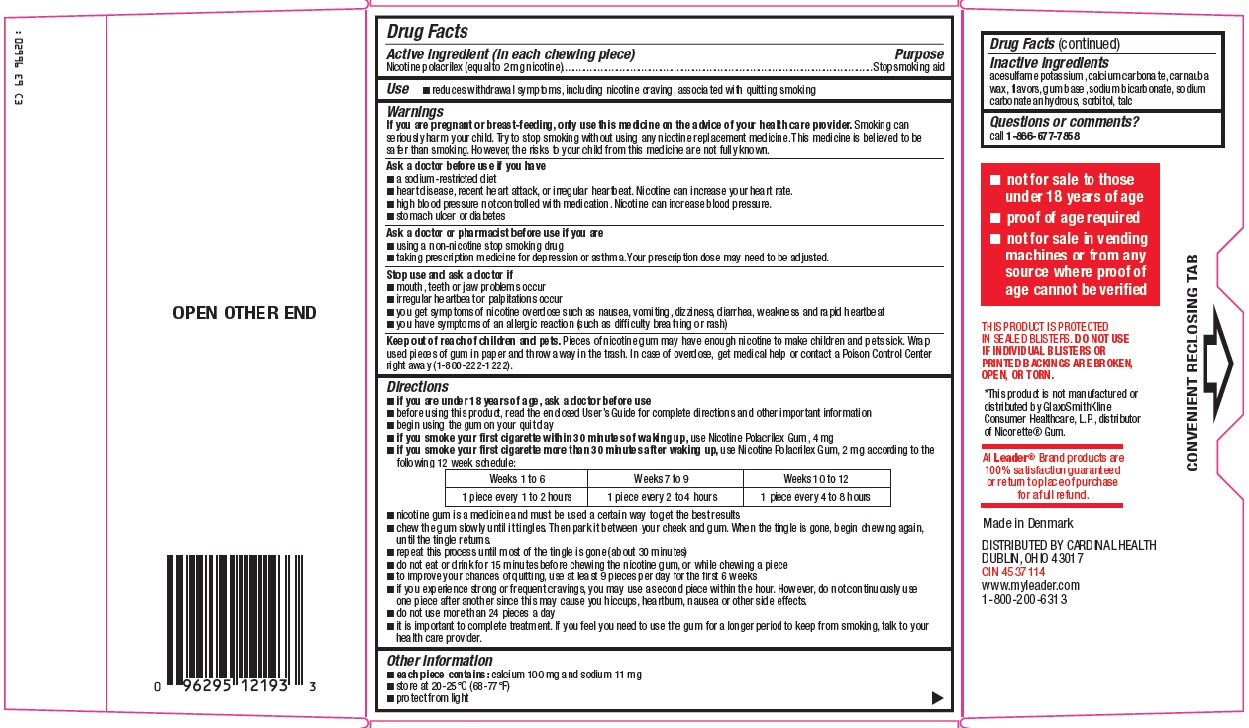
PRINCIPAL DISPLAY PANEL
Compare to Nicorette® Gum active ingredient
Nicotine Polacrilex Gum 2 mg (nicotine)
Stop Smoking Aid
FOR THOSE WHO SMOKE THEIR FIRST CIGARETTE MORE THAN 30 MINUTES AFTER WAKING UP.
If you smoke your first cigarette WITHIN 30 MINUTES of waking up, use Nicotine Polacrilex Gum, 4 mg
Original Flavor
2 mg
Actual Size
NEW DIRECTIONS FOR USE
Keep Using if You Slip Up and Have a Cigarette
Use Beyond 12 Weeks if Needed to Quit
170 PIECES, 2 mg EACH