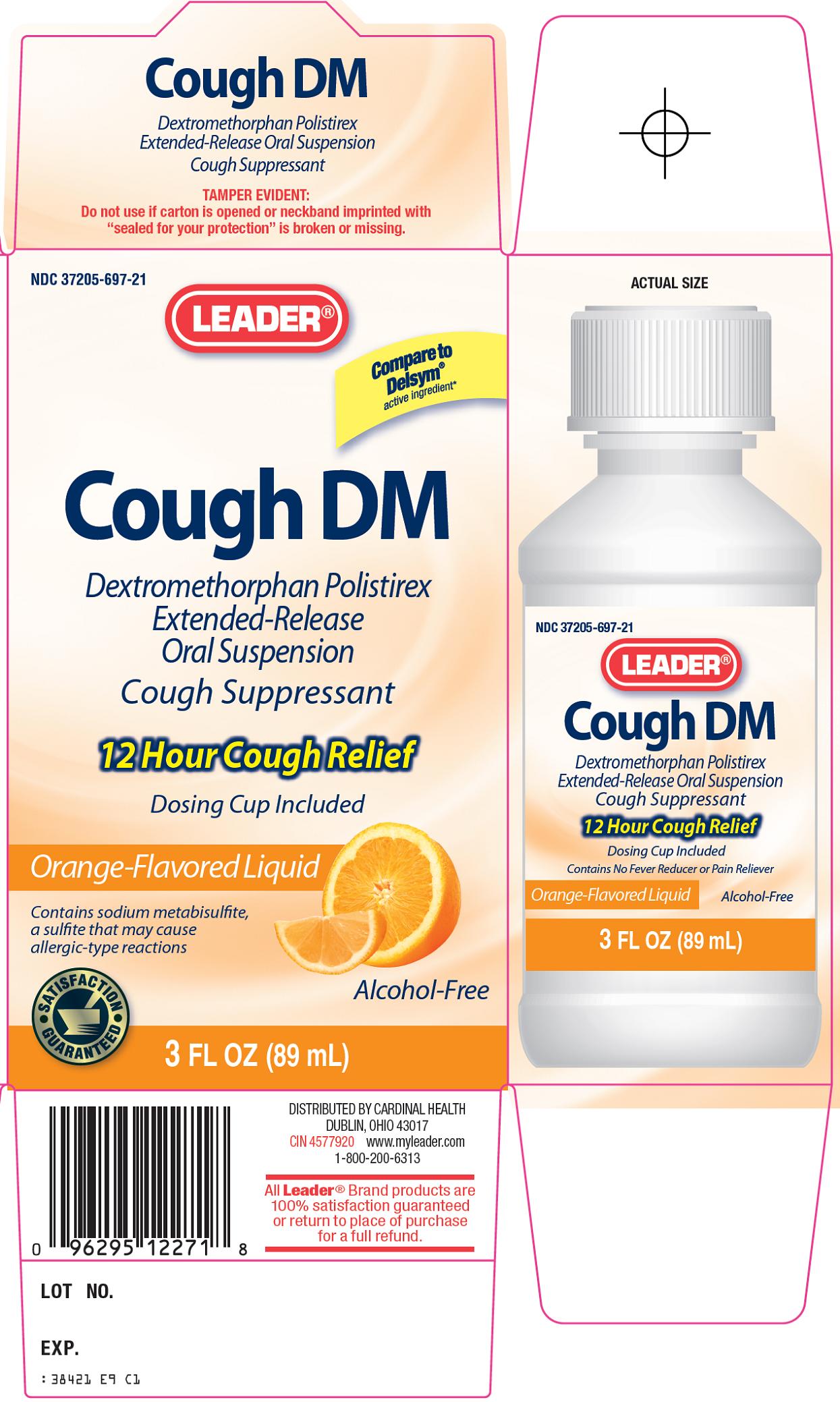
NDC Code(s) : 37205-697-21
Packager : Cardinal Health
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| leader cough dmdextromethorphan polistirex SUSPENSION | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Compare to Delsym® active ingredient
Cough DM
Dextromethorphan Polistirex Extended-Release Oral Suspension
Cough Suppressant
12 Hour Cough Relief
Dosing Cup Included
Orange-Flavored Liquid
Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions
Alcohol-Free