NDC Code(s) : 37205-757-76, 37205-757-87
Packager : Cardinal Health (Leader) 37205
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Aspirin Aspirin TABLET, COATED | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Compare to Bayer® Low Dose Aspirin† active ingredient
‡Aspirin Regimen
Aspirin 81 mg
Pain Reliever (NSAID)
Low Dose Safety Coated
ENTERIC COATED TABLETS
†This product is not manufactured or distributed by Bayer HealthCare LLC, owner of the registered trademark Bayer® Low Dose Aspirin.
‡Aspirin is not appropriate for everyone, so be sure you talk to your doctor before you begin an aspirin regimen.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
PRINCIPAL DISPLAY PANEL
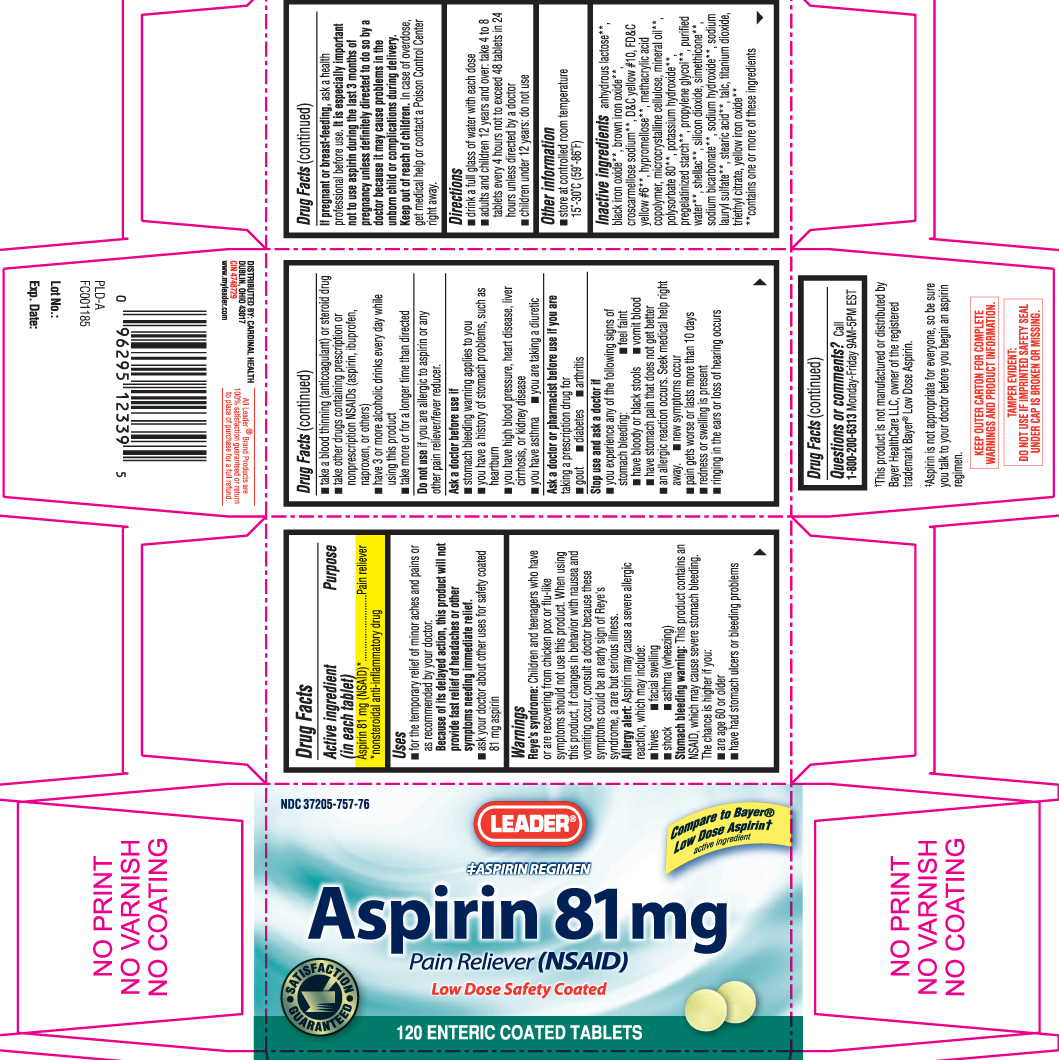 Leader Aspirin 81 mg Low Dose Safety Coated Tablet
Leader Aspirin 81 mg Low Dose Safety Coated Tablet









