NDC Code(s) : 39822-1077-1
Packager : XGen Pharmaceuticals DJB, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| VoriconazoleVoriconazole INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - XGen Pharmaceuticals DJB, Inc.(117380305) |
PRINCIPAL DISPLAY PANEL
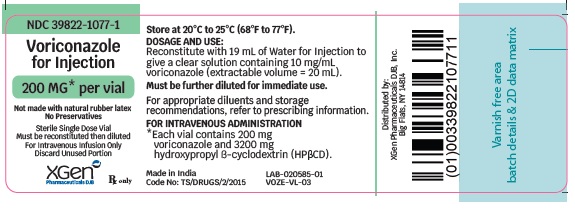
PRINCIPAL DISPLAY PANEL - 200 mg Vial Label
NDC 39822-1077-1
VORICONAZOLE FOR INJECTION
200 MG* per vial
Not made with natural rubber latex
No Preservatives
Sterile Single Dose Vial
Must be reconstituted then diluted
For Intravenous Infusion Only
Discard Unused Portion
Rx only
PRINCIPAL DISPLAY PANEL - 200 mg Vial Carton
NDC 39822-1077-1
VORICONAZOLE FOR INJECTION
200 MG* per vial
Sterile Single Dose Vial
Must be reconstituted then diluted
For Intravenous Infusion Only
Discard Unused Portion
Not made with natural rubber latex
No Preservatives
Rx Only
1 Vial










