NDC Code(s) : 40032-022-02, 40032-022-05, 40032-022-30, 40032-022-31
Packager : Novel Laboratories, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| LinezolidLinezolid TABLET | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Novel Laboratories, Inc.(793518643) |
| REGISTRANT - Novel Laboratories, Inc.(793518643) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Novel Laboratories, Inc. | 793518643 | ANALYSIS(40032-022), MANUFACTURE(40032-022) | |
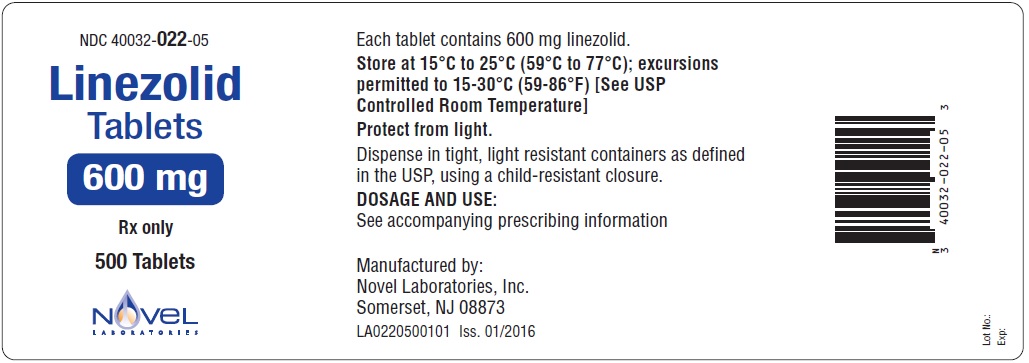
PRINCIPAL DISPLAY PANEL
Linezolid Tablets, 600 mg
Container Label
20 Count

500 Count
30 Units Dose Tablets - Carton Label










