NDC Code(s) : 41163-194-08
Packager : United Natural Foods, Inc. dba UNFI
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| AllergyChlorpheniramine Maleate TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - United Natural Foods, Inc. dba UNFI(943556183) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 038154464 | pack(41163-194) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 832867837 | manufacture(41163-194), pack(41163-194) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 868734088 | manufacture(41163-194) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 967626305 | pack(41163-194) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 117025878 | manufacture(41163-194) | |
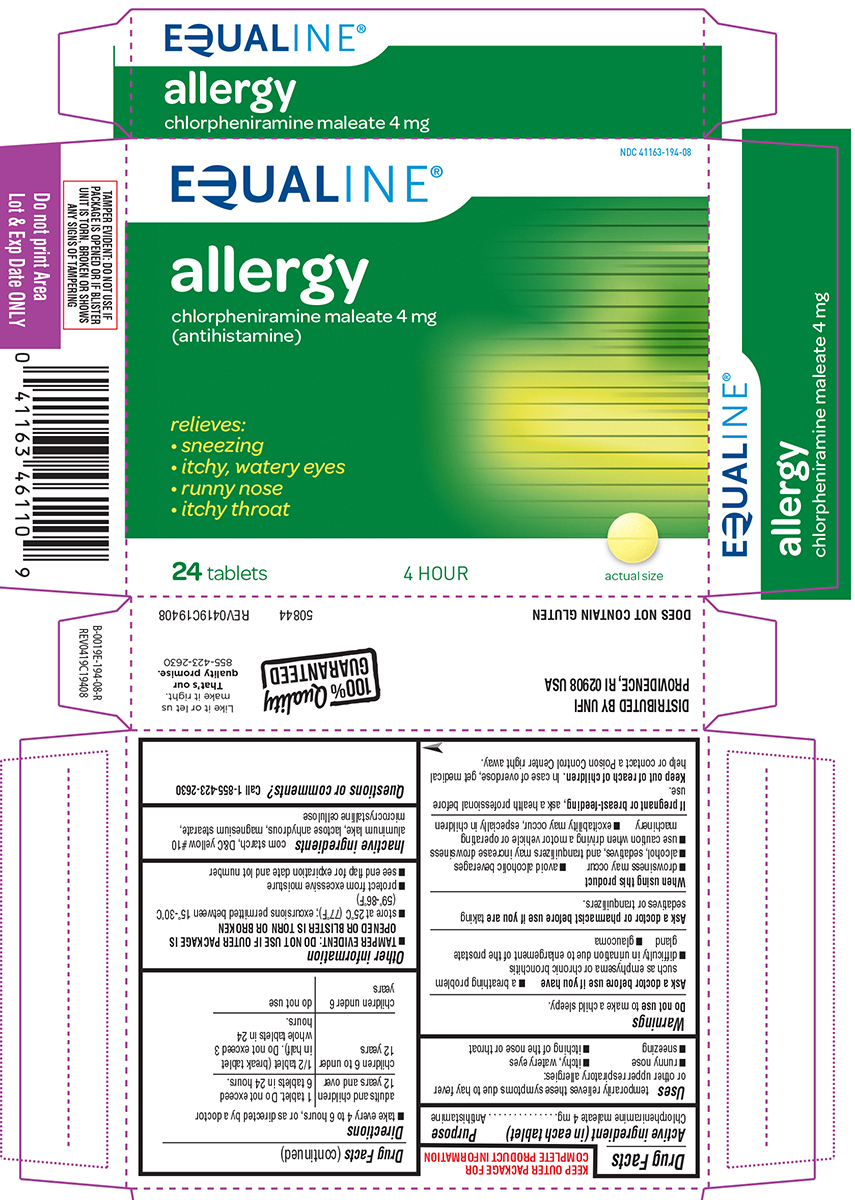
PRINCIPAL DISPLAY PANEL
EQUALINE ®
NDC 41163-194-08
allergy
chlorpheniramine maleate 4 mg
(antihistamine)
relieves:
• sneezing
• itchy, watery eyes
• runny nose
• itchy throat
24 tablets 4 HOUR actual size
TAMPER EVIDENT: DO NOT USE IF
PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS
ANY SIGNS OF TAMPERING
50844 REV0419C19408
DISTRIBUTED BY UNFI
PROVIDENCE, RI 02908 USA
DOES NOT CONTAIN GLUTEN
100% Quality GUARANTEED
Like it or let us
make it right.
That's our
quality promise.
855-423-2630
 44-194
44-194









