NDC Code(s) : 41163-945-32, 41163-945-06
Packager : United Natural Foods, Inc. dba UNFI
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Aspirin Aspirin TABLET, DELAYED RELEASE | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LABELER - United Natural Foods, Inc. dba UNFI(943556183) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 038154464 | pack(41163-945) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 832867837 | manufacture(41163-945) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 832867894 | manufacture(41163-945) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 868734088 | manufacture(41163-945) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 967626305 | pack(41163-945) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 117025878 | manufacture(41163-945) | |
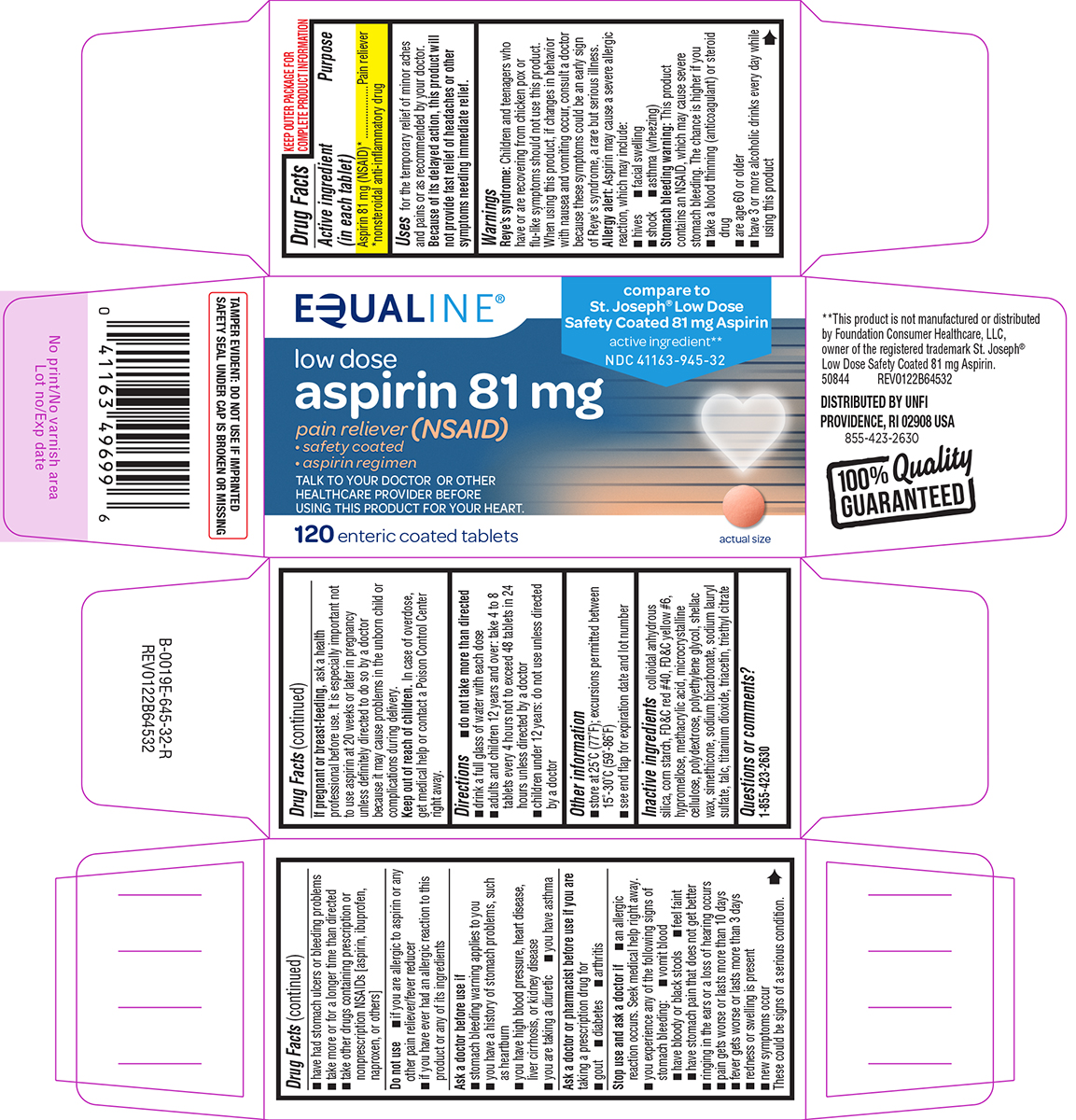
PRINCIPAL DISPLAY PANEL
EQUALINE®
compare to
St. Joseph® Low Dose
Safety Coated 81 mg Aspirin
active ingredient**
NDC 41163-945-32
low dose
aspirin 81 mg
pain reliever (NSAID)
• safety coated
• aspirin regimen
TALK TO YOUR DOCTOR OR OTHER
HEALTHCARE PROVIDER BEFORE
USING THIS PRODUCT FOR YOUR HEART
120 enteric coated tablets
actual size
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
**This product is not manufactured or distributed
by Foundation Consumer Healthcare, LLC,
owner of the registered trademark St. Joseph®
Low Dose Safety Coated 81 mg Aspirin.
50844 REV0122B64532
DISTRIBUTED BY UNFI
PROVIDENCE, RI 02908 USA
855-423-2630
 Equaline 44-645
Equaline 44-645









