NDC Code(s) : 41167-0675-0
Packager : Chattem, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Gold Bond Ultimate Healing Concentrated TherapyWhite Petrolatum OINTMENT | ||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
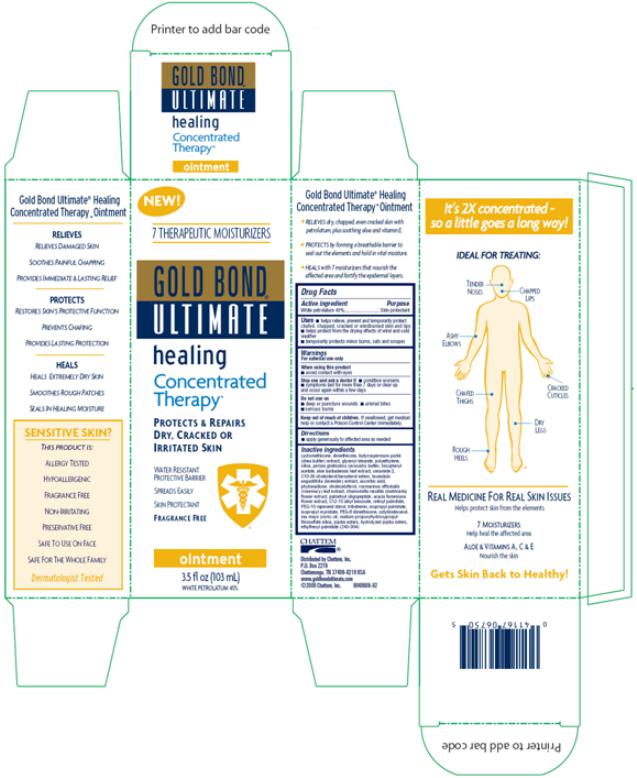
PRINCIPAL DISPLAY PANEL
NDC
41167-
0
6
7
5-
0
NEW!
7 THERAPEUTIC MOISTURIZERS
GOLD BOND
®
ULTIMATE
healing
Concentrated
Therapy
TM
PROTECTS & REPAIRS
DRY, CRACKED OR
IRRITATED SKIN
WATER RESISTANT
PROTECTIVE BARRIER
SPREADS EASILY
SKIN PROTECTANT
FRAGRANCE FREE
ointment
3.5 fl oz (103 mL)
WHITE PETROLATUM 45
%