NDC Code(s) : 42192-108-04
Packager : Acella Pharmaceuticals, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Acella Hydrocortisone Acetate - Pramoxine SinglesHYDROCORTISONE ACETATE, PRAMOXINE CREAM | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
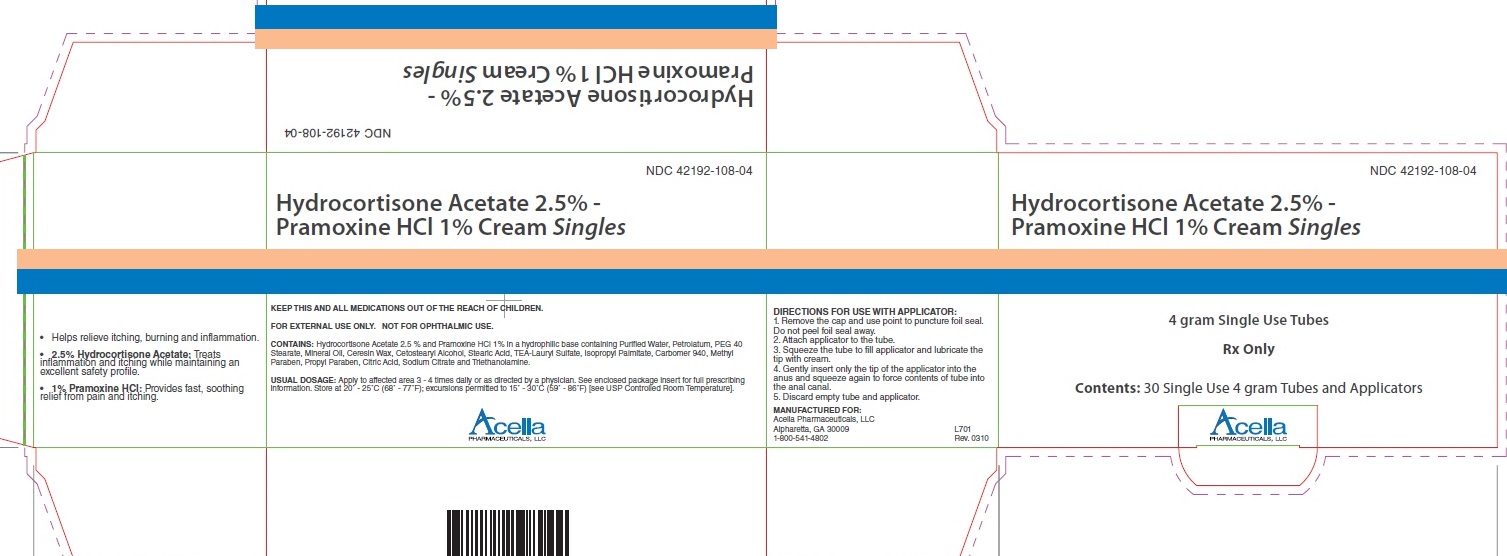
PRINCIPAL DISPLAY PANEL
NDC 42192-108-04 Rx Only Net Wt. 4 g
Hydrocortisone Acetate 2.5% -
Pramoxine HCl 1% Cream
CONTAINS: Hydrocortisone Acetate 2.5% and Pramoxine HCl 1% in a hydrophilic base containing Purified Water, Petrolatum, PEG 40 Stearate, Mineral Oil, Ceresin Wax, Cetostearyl Alcohol, Stearic Acid, TEA-Lauryl Sulfate, Isopropyl Palmitate, Carbomer Homopolymer Type C, Methyl Paraben, Propyl Paraben, Citric Acid, Sodium Citrate and Triethanolamine.
USUAL DOSAGE: Apply to affected area 3 - 4 times daily. See enclosed package insert for full prescribing information.
FOR EXTERNAL USE ONLY. NOT FOR OPTHALMIC USE.
Store at controlled room temperature 15 degree - 30 degree C (59 degree - 86 degree F) Keep tightly closed.
TO OPEN: Remove cap and use point to puncture foil seal.
MANUFACTURED FOR: Acella Pharmaceuticals, LLC L697
Alpharetta, GA 30009 Rev. 0610
PRINCIPAL DISPLAY PANEL
- Helps relieve itching, burning and inflammation.
- 2.5% Hydrocortisone Acetate: Treats inflammation and itching while maintaining an excellent safety profile.
- 1% Pramoxine HCl: Provides fast, soothing relief from pain and itching.
NDC 42192-108-04
Hydrocortisone Acetate 2.5% -
Pramoxine HCl 1% Cream Singles
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE.
CONTAINS: Hydrocortisone Acetate 2.5% and Pramoxine HCl 1% in a hydrophilic base containing Purified Water, Petrolatum, PEG 40 Stearate, Mineral Oil, Ceresin Wax, Cetostearyl Alcohol, Stearic Acid, TEA-Lauryl Sulfate, Isopropyl Palmitate, Carbomer 940, Methyl Paraben, Propyl Paraben, Citric Acid, Sodium Citrate and Triethanolamine.
USUAL DOSAGE: Apply to affected area 3 - 4 times daily or as directed by a physician. See enclosed package Insert for full prescribing information. Store at 20 degree - 25 degree C (68 degree - 77 degree F) ; excursions permitted to 15 degree - 30 degree C (59 degree - 86 degree F) [see USP Controlled Room Temperature].
Acella Pharmaceuticals, LLC
NDC 42192-108-04
Hydrocortisone Acetate 2.5% -
Pramoxine HCl 1% Cream Singles
DIRECTIONS FOR USE WITH APPLICATOR:
1. Remove the cap and use point to puncture foil seal. Do not peel foil seal away.
2. Attach applicator to the tube.
3. Squeeze the tube to fill applicator and lubricate the tip with cream.
4. Gently insert only the tip of the applicator into the anus and squeeze again to
force contents of tube into the anal canal.
5. Discard empty tube and applicator.
MANUFACTURED FOR:
Acella Pharmaceuticals, LLC
Alpharetta, GA 30009 L701
1-800-541-4902 Rev. 0310
NDC 42192-108-04
Hydrocortisone Acetate 2.5% -
Pramoxine HCl 1% Cream Singles
4 gram Single Use Tubes
Rx Only
Contents: 30 Single Use 4 gram Tubes and Applicators
Acella Pharmaceuticals, LLC
PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL