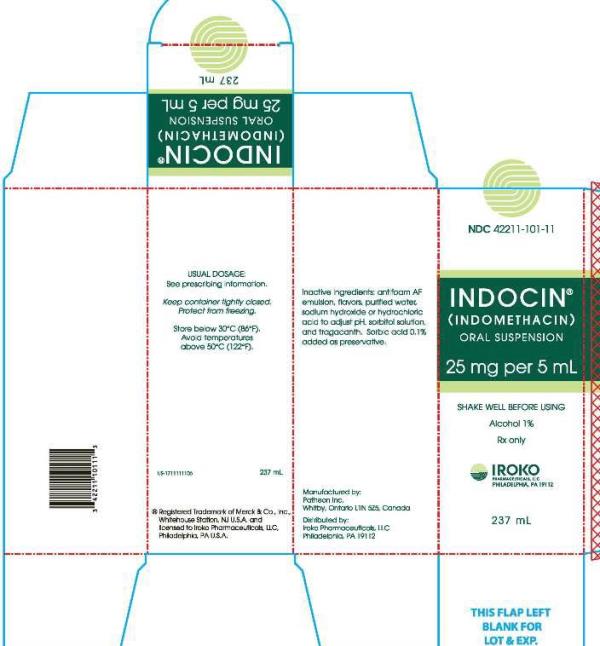
NDC Code(s) : 42211-101-11, 42211-101-01
Packager : Iroko Pharmaceuticals, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| INDOCINindomethacin SUSPENSION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Iroko Pharmaceuticals, LLC(796831217) |
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
INDOCIN
(INDOMETHACIN)
ORAL SUSPENSION
25 mg per 5 mL
237 mL