NDC Code(s) : 42283-002-00
Packager : Laboratorios Genesse SL
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Lactovit Original Roll-On Antiperspirant Deodorant Aluminum Chlorohydrate LIQUID | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
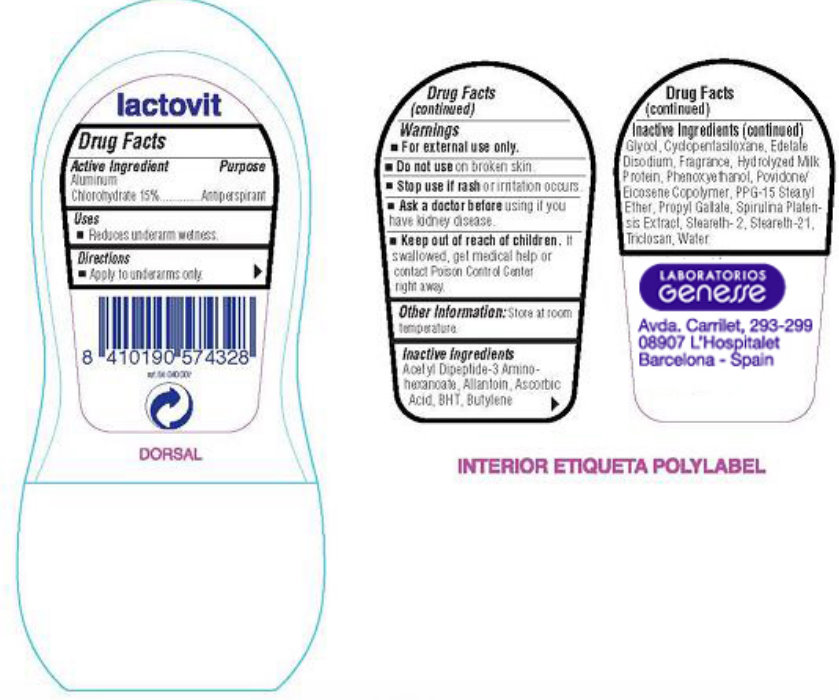
PRINCIPAL DISPLAY PANEL
lactovit original original Roll-On Antiperspirant Deodorant Care and nutrition on your skin NET WT 1.7 FL. OZ (50 ml).
PRINCIPAL DISPLAY PANEL
lactovit DORSAL LABORATORIOS GENESSE Avda, Carrilet, 293-299 08907 L'Hospitalet Barcelona - Spain
PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL