NDC Code(s) : 42385-972-30, 42385-972-90, 42385-972-01, 42385-972-05, 42385-972-11, 42385-972-72, 42385-973-30, 42385-973-90, 42385-973-01, 42385-973-05, 42385-973-11, 42385-973-72, 42385-974-30, 42385-974-90, 42385-974-01, 42385-974-05, 42385-974-11, 42385-974-72
Packager : Laurus Labs Limited
Category : Human Prescription Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Gabapentin Gabapentin CAPSULE | |||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Gabapentin Gabapentin CAPSULE | |||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Gabapentin Gabapentin CAPSULE | |||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Laurus Labs Limited(915075687) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Laurus Labs Limited (VSP2) | 650885309 | ANALYSIS(42385-972, 42385-973, 42385-974), MANUFACTURE(42385-972, 42385-973, 42385-974) | |
PRINCIPAL DISPLAY PANEL
NDC 42385-972-30
Gabapentin
Capsules, USP
100 mg
Dispense the enclosed
Medication Guide to
each patient.
30 Capsules Rx only
LAURUSLABS
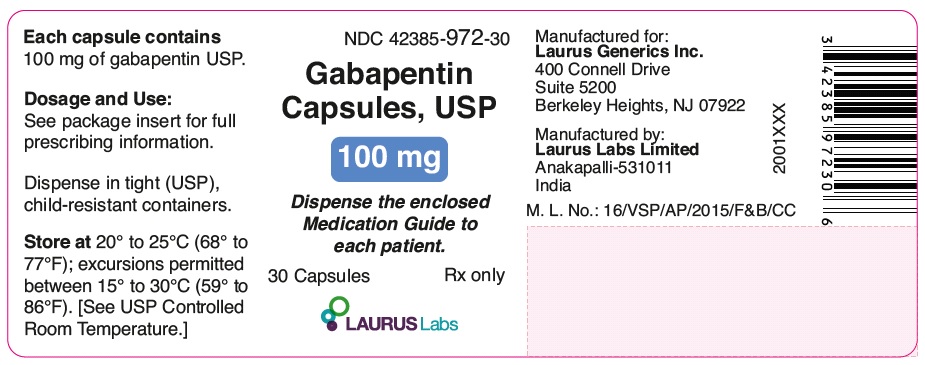
PRINCIPAL DISPLAY PANEL
Rx only NDC 42385-972-17
Gabapentin Capsule, USP
100 mg
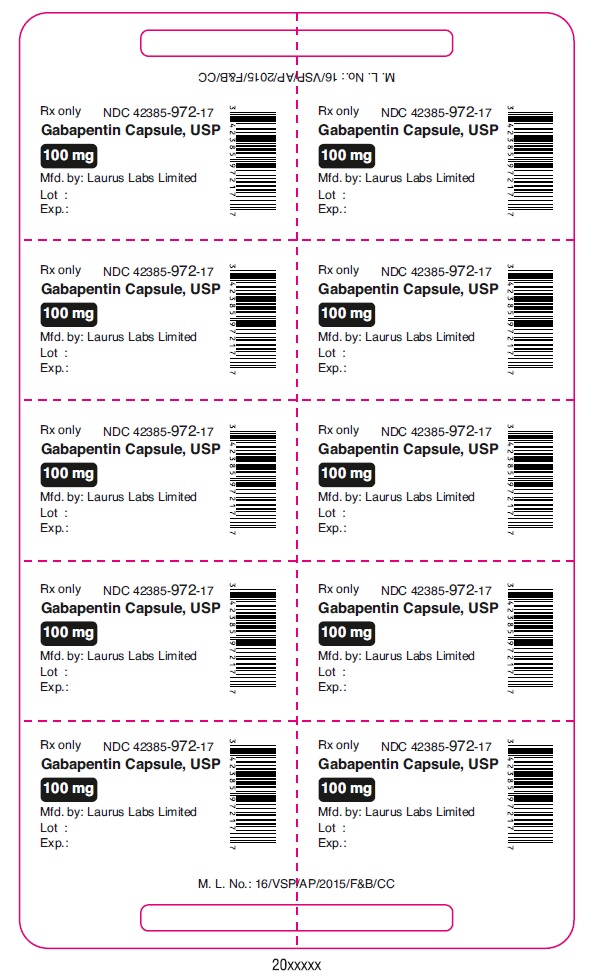
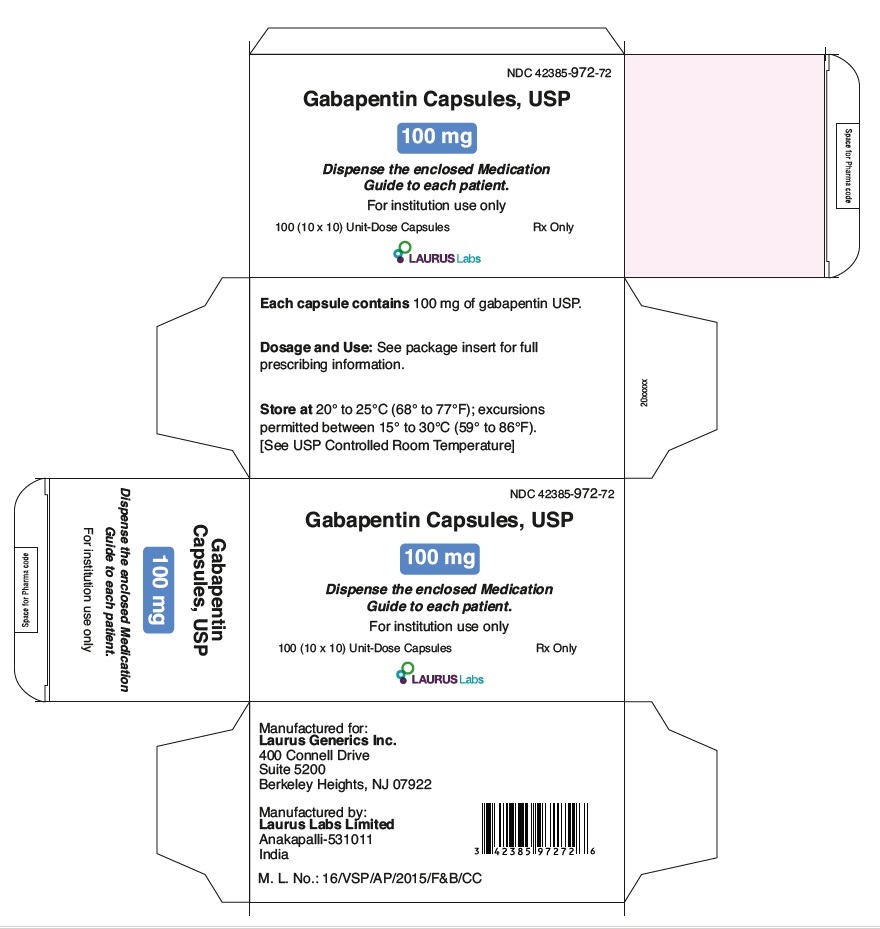
PRINCIPAL DISPLAY PANEL
NDC 42385-972-72
Gabapentin Capsules, USP
100 mg
Dispense the enclosed Medication
Guide to each patient
For institution use only
100 (10 x 10) Unit-Dose Capsules Rx Only
LAURUSLABS
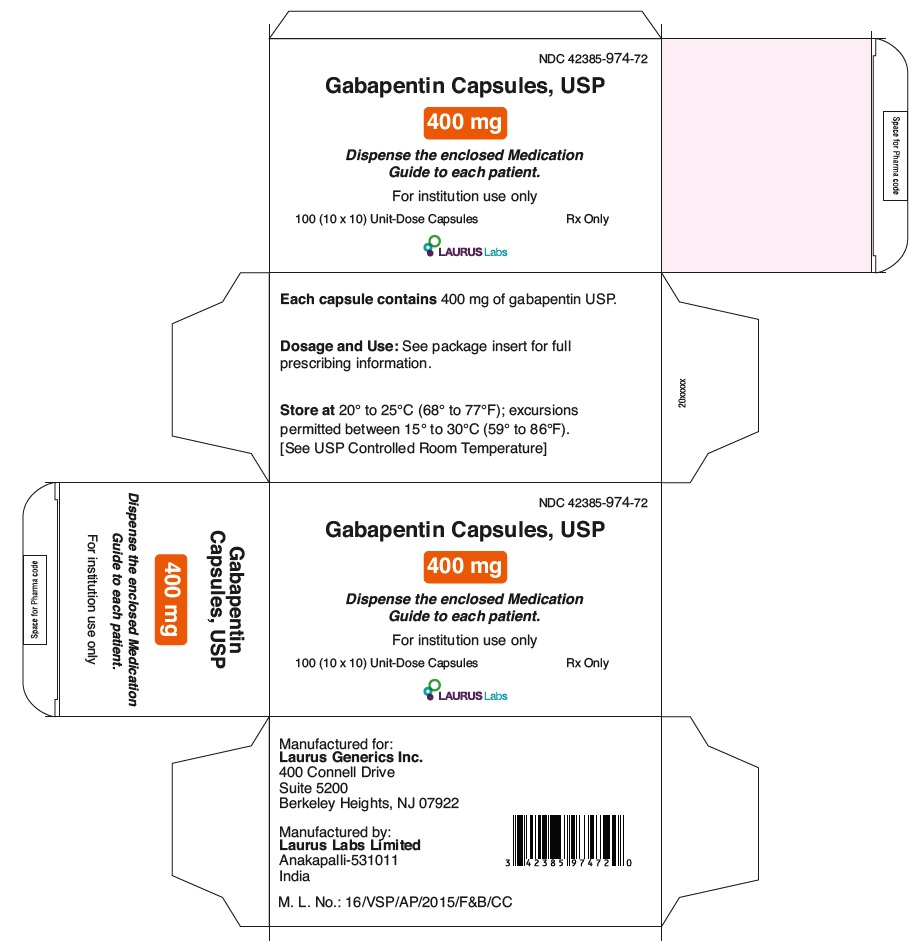
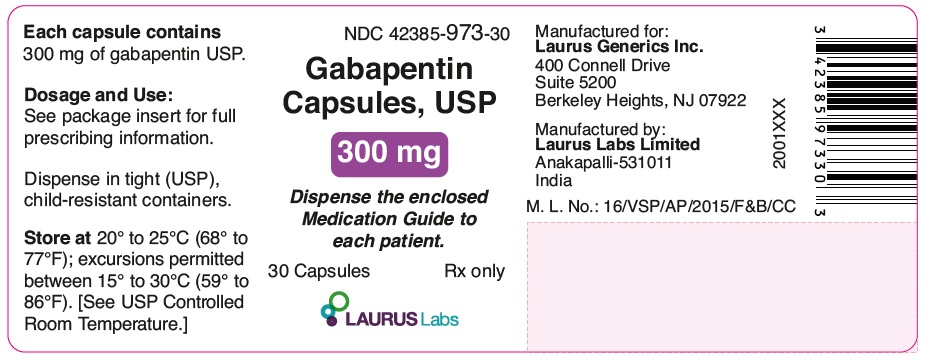
PRINCIPAL DISPLAY PANEL
NDC 42385-973-30
Gabapentin
Capsules, USP
300 mg
Dispense the enclosed
Medication Guide to each patient.
30 Capsules Rx only
LAURUSLABS
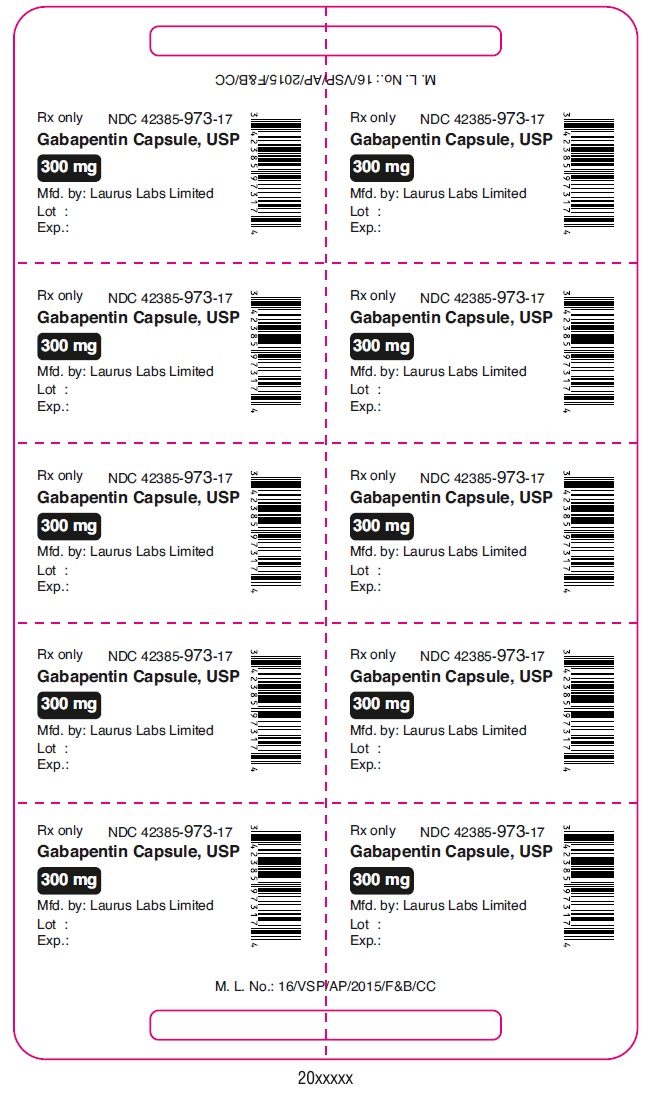
PRINCIPAL DISPLAY PANEL
Rx only NDC 42385-973-17
Gabapentin Capsule, USP
300 mg
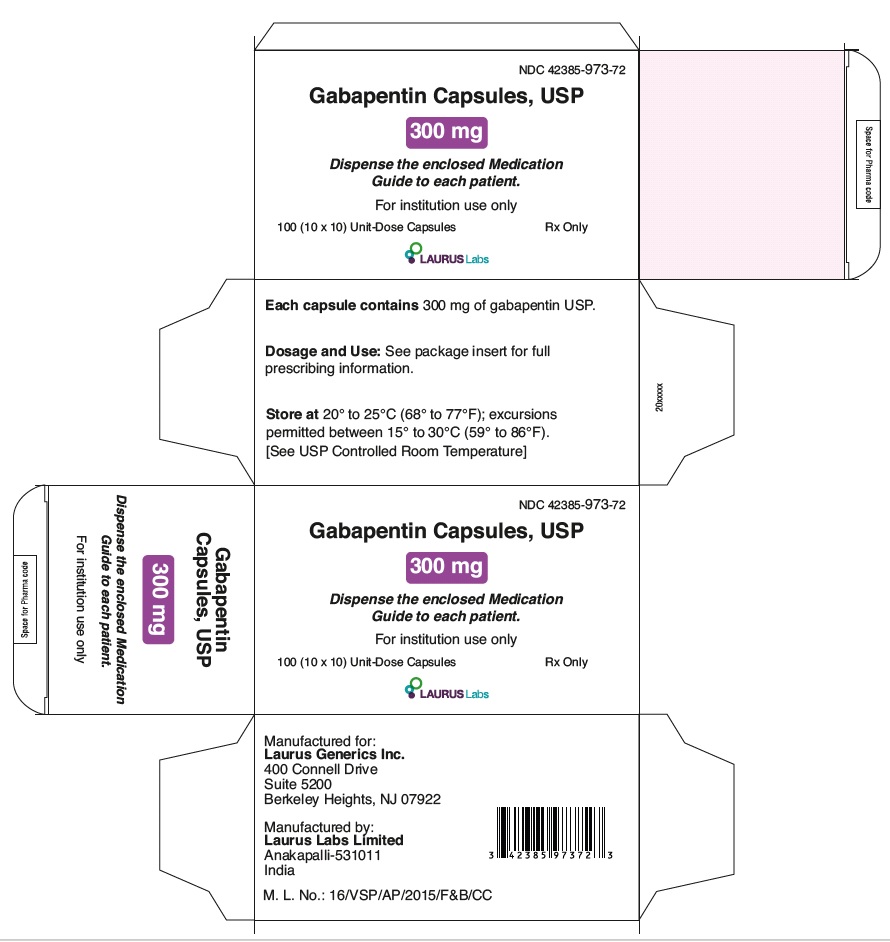
PRINCIPAL DISPLAY PANEL
NDC 42385-973-72
Gabapentin Capsules, USP
300 mg
Dispense the enclosed Medication
Guide to each patient.
For institution use only
100 (10 x 10) Unit-Dose Capsules Rx Only
LAURUSLABS
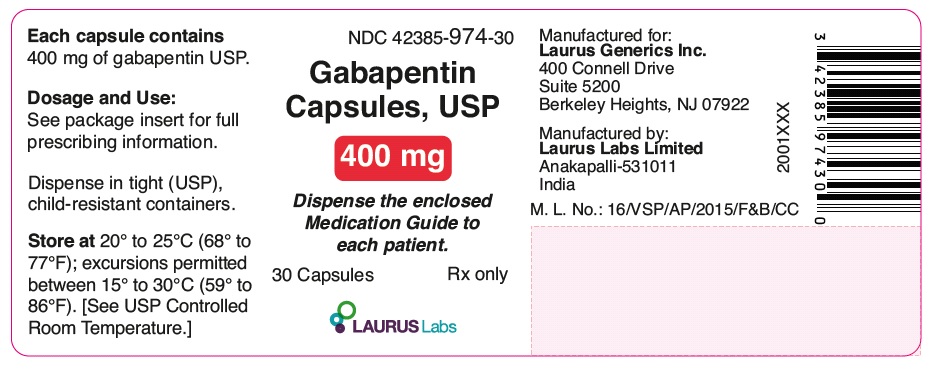
PRINCIPAL DISPLAY PANEL
NDC 42385-974-30
Gabapentin
Capsules, USP
400 mg
Dispense the enclosed
Medication Guide to each patient.
30 Capsules Rx only
LAURUSLABS
PRINCIPAL DISPLAY PANEL
Rx only NDC 42385-974-17
Gabapentin Capsule, USP
400 mg
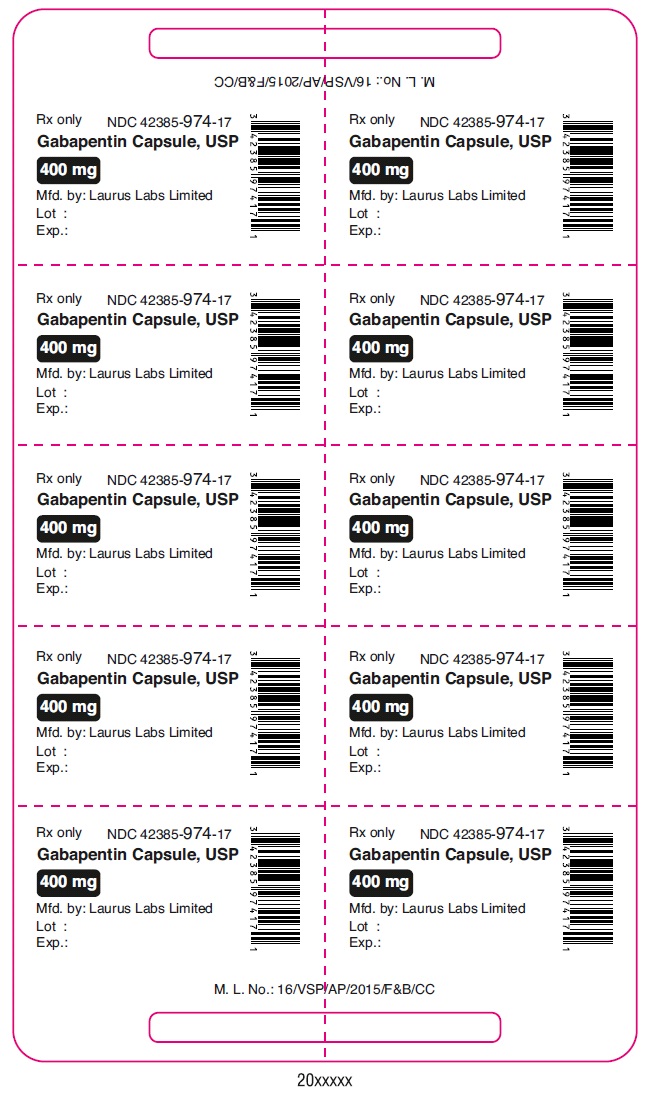
PRINCIPAL DISPLAY PANEL
NDC 42385-974-72
Gabapentin Capsules, USP
400 mg
Dispense the enclosed Medication
Guide to each patient.
For institution use only
100 (10 x 10) Unit-Dose Capsules Rx Only
LAURUSLABS