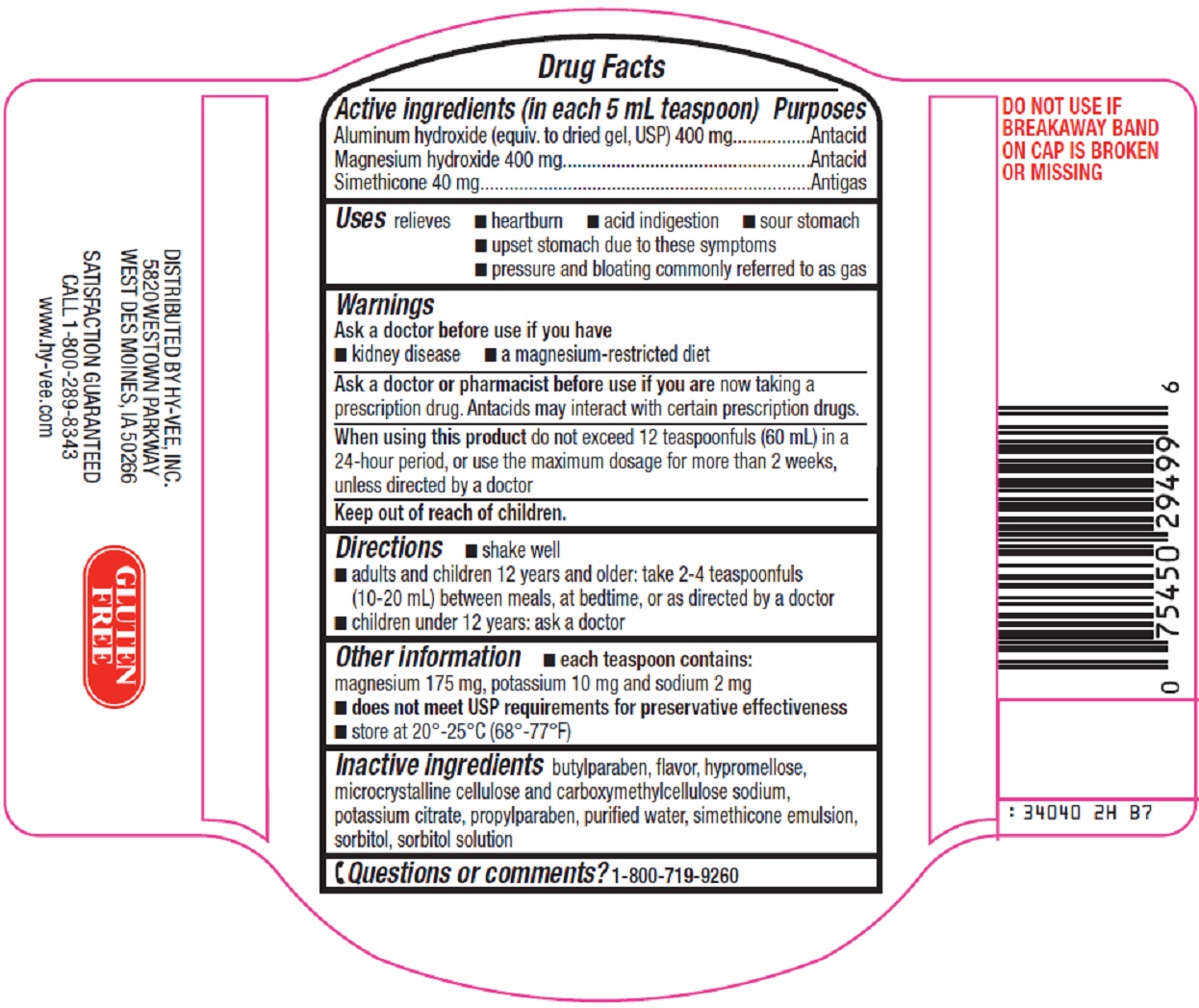
NDC Code(s) : 42507-340-40
Packager : HyVee Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| antacidaluminum hydroxide, magnesium hydroxide, simethicone LIQUID | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Compare to Mylanta® Maximum Strength active ingredients
Maximum Strength
Antacid/Anti-Gas
Antacid
Fast Acting
Soothing Relief of
Heartburn
Acid Indigestion
Sour Stomach
Classic Original Flavor
12 FL OZ (355 mL)