NDC Code(s) : 42508-265-09
Packager : Arbonne International, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Arbonne RE9 Advanced for Men Octisalate, Octocrylene, and Zinc Oxide LOTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
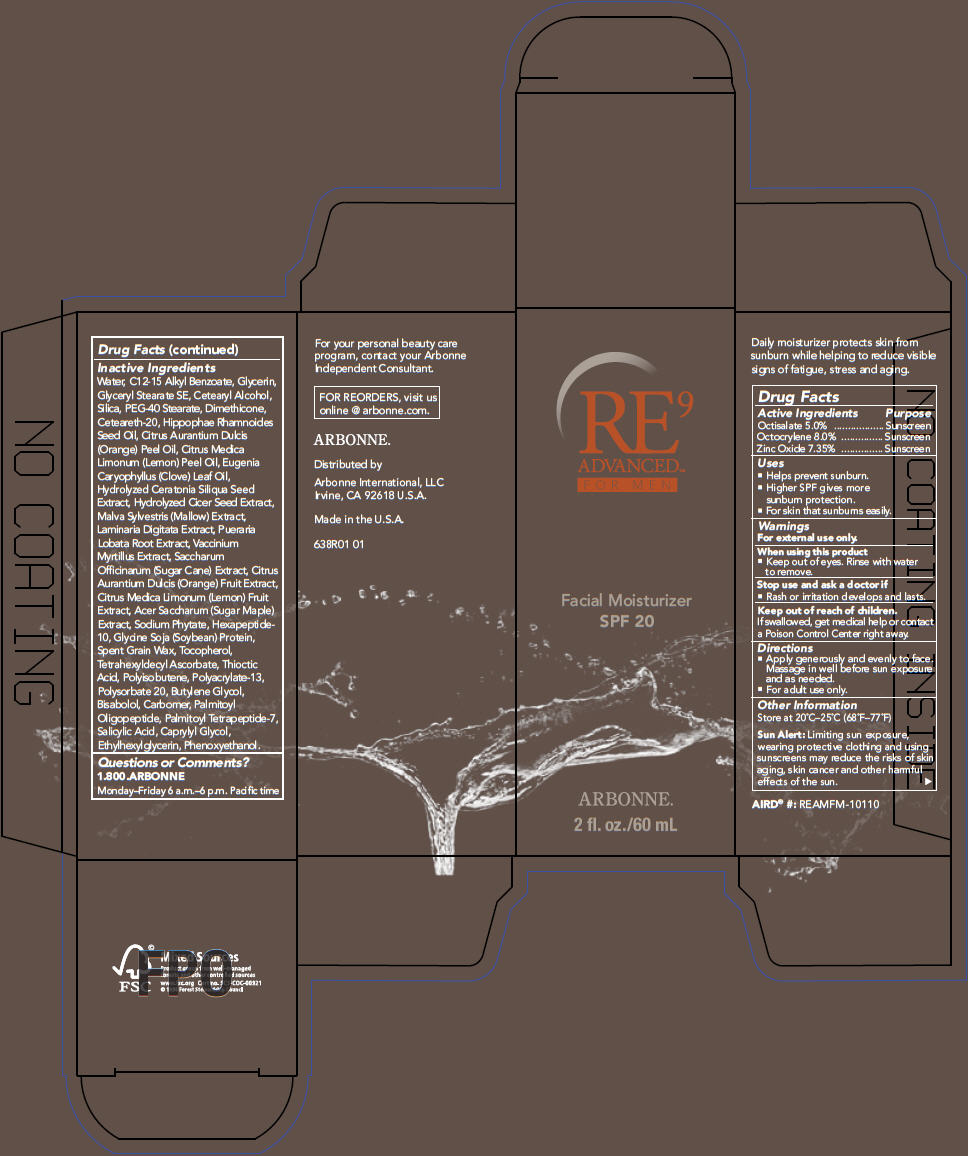
PRINCIPAL DISPLAY PANEL
RE9
ADVANCED™
FOR MEN
Facial Moisturizer
SPF 20
ARBONNE ®
2 fl. oz./60 mL