NDC Code(s) : 42508-299-08, 42508-299-15, 42508-299-11
Packager : Arbonne International, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Arbonne RE9 Advanced AVOBENZONE, HOMOSALATE, OCTINOXATE, and OCTOCRYLENE LOTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
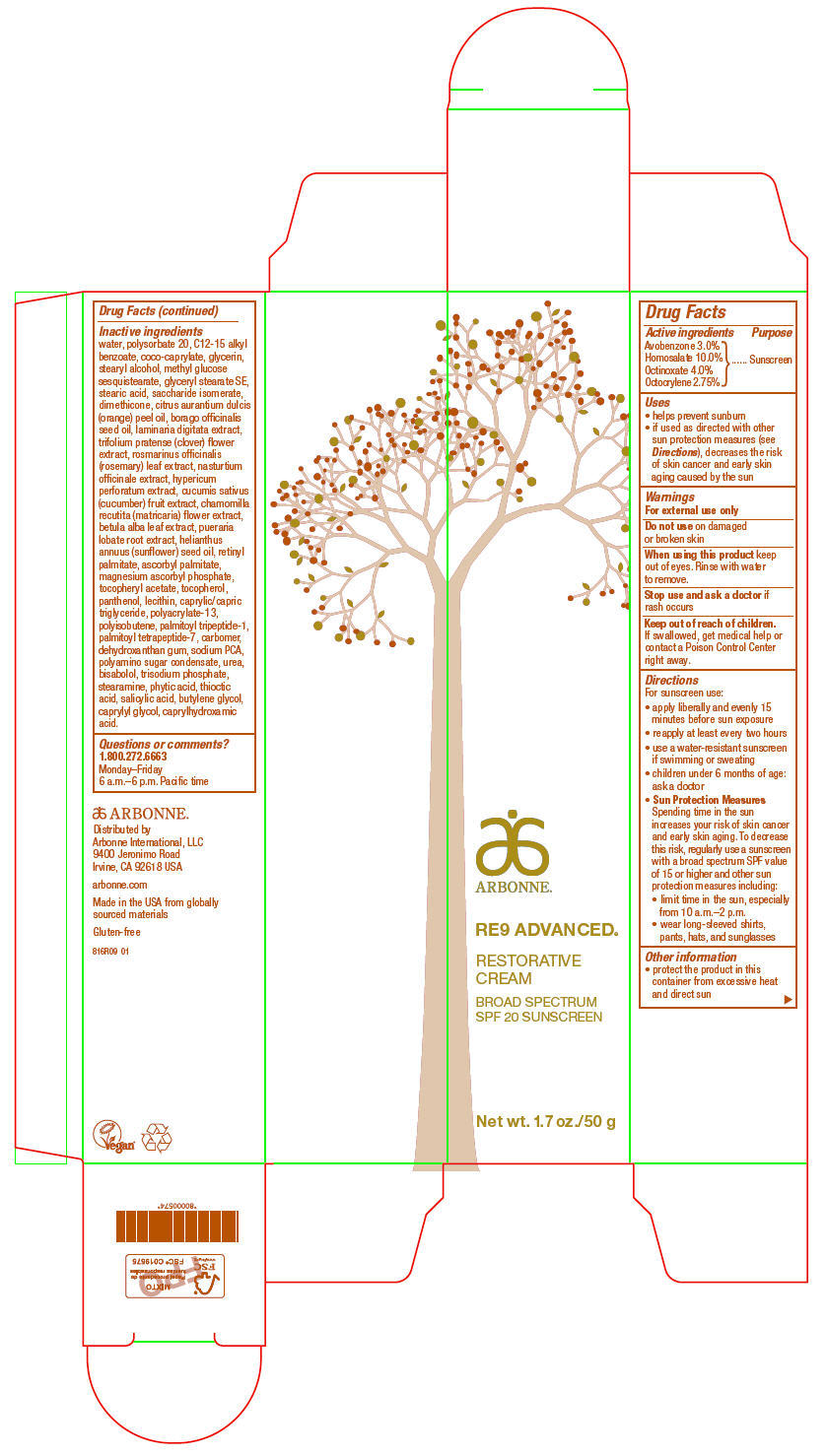
PRINCIPAL DISPLAY PANEL
ARBONNE®
RE9 ADVANCED®
RESTORATIVE
CREAM
BROAD SPECTRUM
SPF 20 SUNSCREEN
Net wt. 1.7 oz./50 g