NDC Code(s) : 42571-160-30, 42571-160-01, 42571-160-32, 42571-160-11, 42571-161-42, 42571-161-01, 42571-161-32, 42571-161-11, 42571-162-42, 42571-162-01, 42571-162-43, 42571-162-44
Packager : Micro Labs Limited
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| AMOXICILLIN AND CLAVULANATE POTASSIUM Amoxicillin and Clavulanate Potassium TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| AMOXICILLIN AND CLAVULANATE POTASSIUM Amoxicillin and Clavulanate Potassium TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| AMOXICILLIN AND CLAVULANATE POTASSIUM Amoxicillin and Clavulanate Potassium TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Micro Labs Limited(862174955) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Micro Labs Limited | 867064609 | analysis(42571-160, 42571-161, 42571-162), label(42571-160, 42571-161, 42571-162), manufacture(42571-160, 42571-161, 42571-162), pack(42571-160, 42571-161, 42571-162) | |
PRINCIPAL DISPLAY PANEL
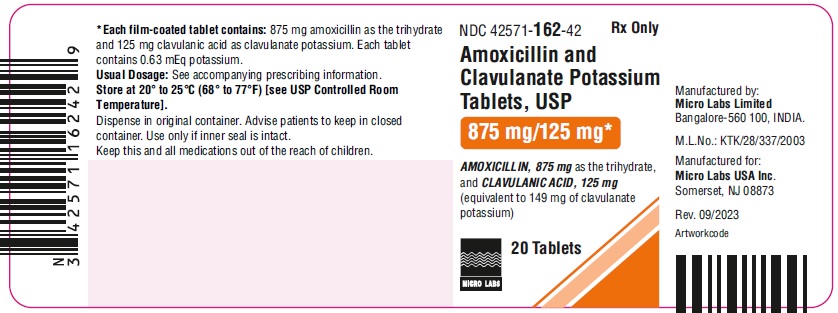
NDC 42571-160-30
Amoxicillin and Clavulanate
Potassium Tablets, USP
250 mg/125 mg*
Rx Only
30 Tablets
MICRO LABS LIMITED
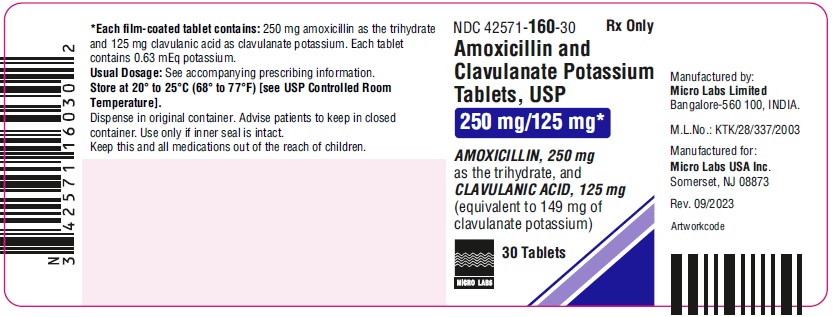
NDC 42571-161-42
Amoxicillin and Clavulante
Potassium Tablets, USP
500 mg/125 mg*
Rx Only
20 Tablets
MICRO LABS LIMITED
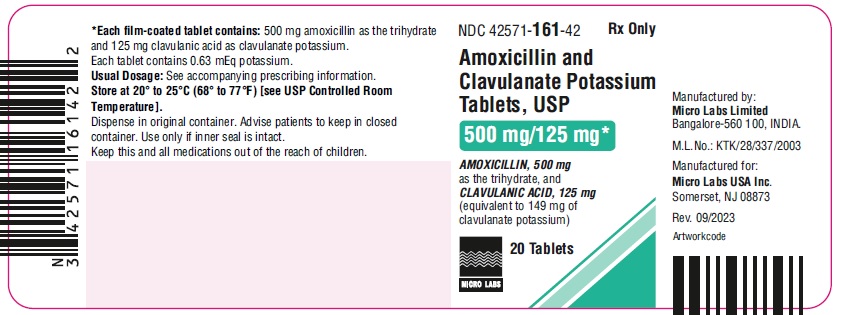
NDC 42571-162-42
Amoxicillin and Clavulanate
Potassium Tablets, USP
875 mg/125 mg*
Rx Only
20 Tablets
MICRO LABS LIMITED