NDC Code(s) : 42808-200-05, 42808-200-09
Packager : Exact-Rx, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| UreaUrea CREAM | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
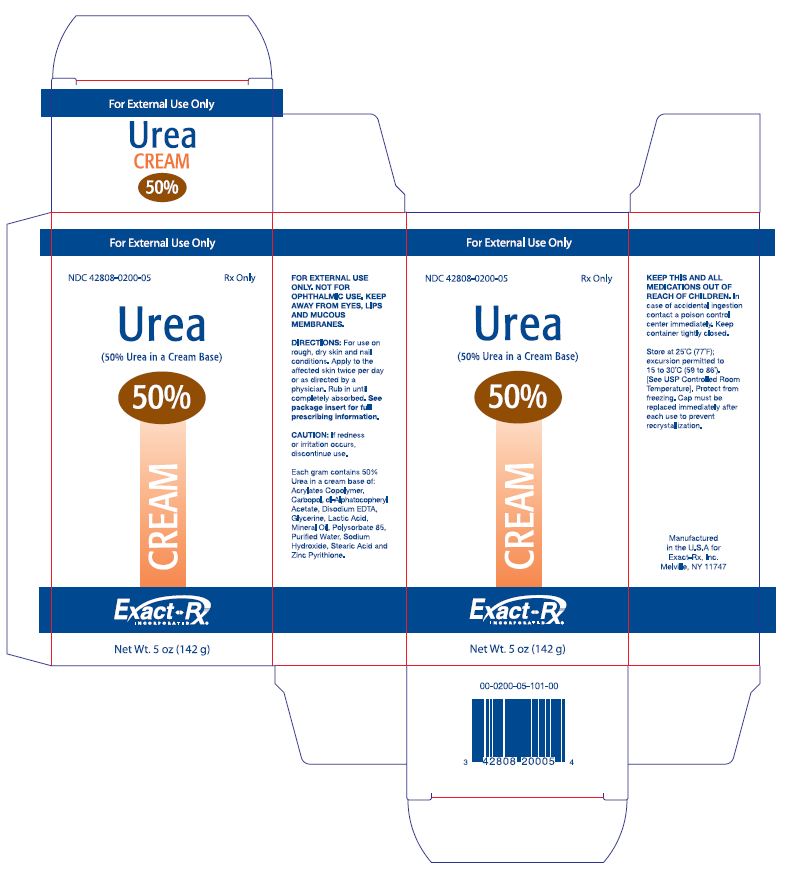
PRINCIPAL DISPLAY PANEL
For External Use Only
NDC 42808-0200-05 Rx Only
Urea
(50% Urea in a Cream Base)
50%
CREAM
Exact-Rx.
INCORPORATED
Net Wt. 5 oz (142 g)