NDC Code(s) : 42858-750-40, 42858-353-40, 42858-493-40, 42858-586-40, 42858-839-40
Packager : Rhodes Pharmaceuticals L.P.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CIII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| BuprenorphineBuprenorphine PATCH | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| BuprenorphineBuprenorphine PATCH | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| BuprenorphineBuprenorphine PATCH | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| BuprenorphineBuprenorphine PATCH | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| BuprenorphineBuprenorphine PATCH | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Rhodes Pharmaceuticals L.P.(831928986) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LTS Lohmann Therapy Systems Corp. | 787660513 | MANUFACTURE(42858-750, 42858-353, 42858-493, 42858-586, 42858-839) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LTS Lohmann Therapie-Systeme AG | 342693590 | MANUFACTURE(42858-750, 42858-353, 42858-493, 42858-586, 42858-839) | |
PRINCIPAL DISPLAY PANEL
Contains:
4 Transdermal Systems
One package of 4 Disposal Units
NDC 42858-750-40
Buprenorphine Transdermal System
5 mcg/hour
CIII
ATTENTION
DISPENSER:
The enclosed
Medication Guide
MUST be provided
to the patient
upon dispensing.
Systemic delivery of 5 mcg per hour of buprenorphine for seven days.
Because serious or life-threatening breathing problems could result,
DO NOT USE BUPRENORPHINE TRANSDERMAL SYSTEM for:
- pain that can be treated with immediate-release opioids or
non-opioid analgesics - intermittent (on an as-needed basis) pain
Rx only

PRINCIPAL DISPLAY PANEL
Contains:
4 Transdermal Systems
One package of 4 Disposal Units
NDC 42858-353-40
Buprenorphine Transdermal System
7.5 mcg/hour
CIII
ATTENTION
DISPENSER:
The enclosed
Medication Guide
MUST be provided
to the patient
upon dispensing.
Systemic delivery of 7.5 mcg per hour of buprenorphine for seven days.
Because serious or life-threatening breathing problems could result,
DO NOT USE BUPRENORPHINE TRANSDERMAL SYSTEM for:
- pain that can be treated with immediate-release opioids or
non-opioid analgesics - intermittent (on an as-needed basis) pain
Rx only
for use in
opioid-experienced
patients only

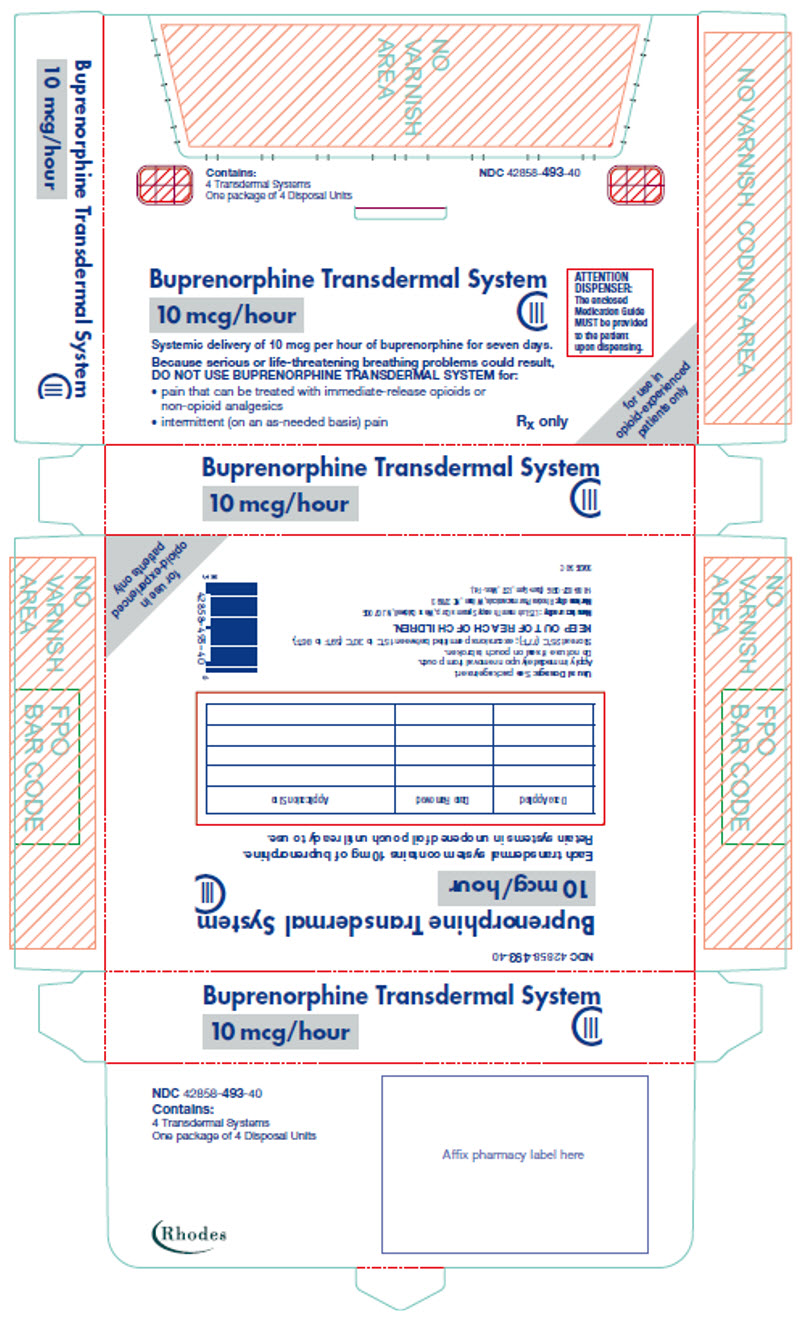
PRINCIPAL DISPLAY PANEL
Contains:
4 Transdermal Systems
One package of 4 Disposal Units
NDC 42858-493-40
Buprenorphine Transdermal System
10 mcg/hour
CIII
ATTENTION
DISPENSER:
The enclosed
Medication Guide
MUST be provided
to the patient
upon dispensing.
Systemic delivery of 10 mcg per hour of buprenorphine for seven days.
Because serious or life-threatening breathing problems could result,
DO NOT USE BUPRENORPHINE TRANSDERMAL SYSTEM for:
- pain that can be treated with immediate-release opioids or
non-opioid analgesics - intermittent (on an as-needed basis) pain
Rx only
for use in
opioid-experienced
patients only
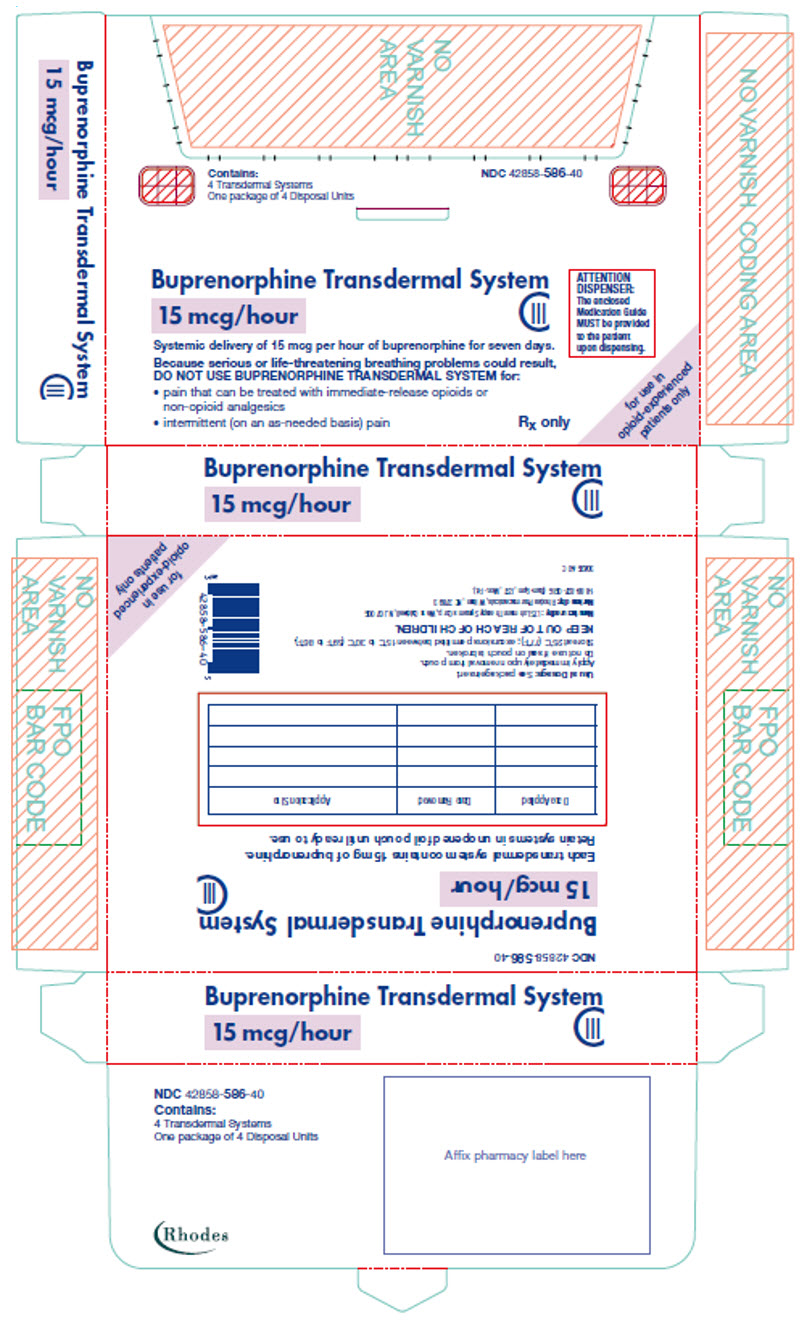
PRINCIPAL DISPLAY PANEL
Contains:
4 Transdermal Systems
One package of 4 Disposal Units
NDC 42858-586-40
Buprenorphine Transdermal System
15 mcg/hour
CIII
ATTENTION
DISPENSER:
The enclosed
Medication Guide
MUST be provided
to the patient
upon dispensing.
Systemic delivery of 15 mcg per hour of buprenorphine for seven days.
Because serious or life-threatening breathing problems could result,
DO NOT USE BUPRENORPHINE TRANSDERMAL SYSTEM for:
- pain that can be treated with immediate-release opioids or
non-opioid analgesics - intermittent (on an as-needed basis) pain
Rx only
for use in
opioid-experienced
patients only
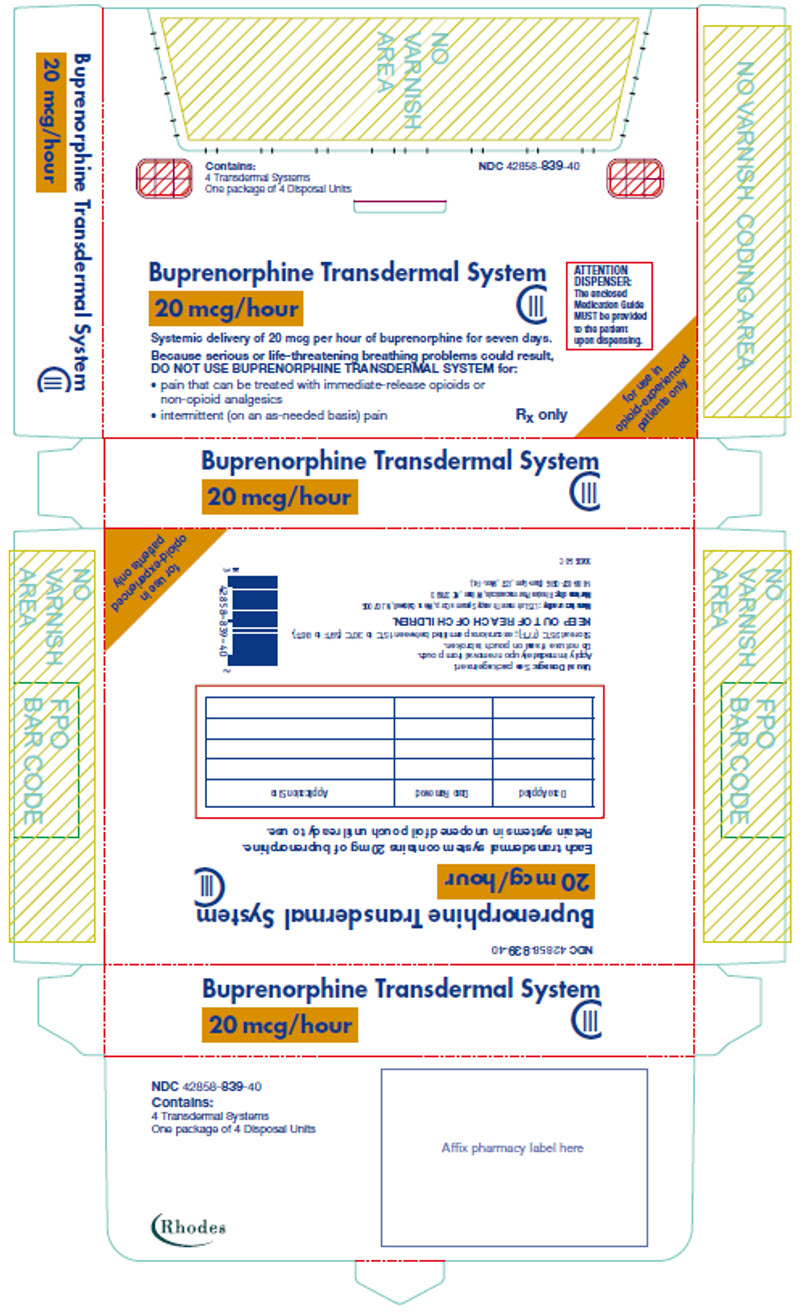
PRINCIPAL DISPLAY PANEL
Contains:
4 Transdermal Systems
One package of 4 Disposal Units
NDC 42858-839-40
Buprenorphine Transdermal System
20 mcg/hour
CIII
ATTENTION
DISPENSER:
The enclosed
Medication Guide
MUST be provided
to the patient
upon dispensing.
Systemic delivery of 20 mcg per hour of buprenorphine for seven days.
Because serious or life-threatening breathing problems could result,
DO NOT USE BUPRENORPHINE TRANSDERMAL SYSTEM for:
- pain that can be treated with immediate-release opioids or
non-opioid analgesics - intermittent (on an as-needed basis) pain
Rx only
for use in
opioid-experienced
patients only