NDC Code(s) : 42874-117-01, 42874-117-10
Packager : Protein Sciences Corporation
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Flublok Quadrivalentinfluenza vaccine INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
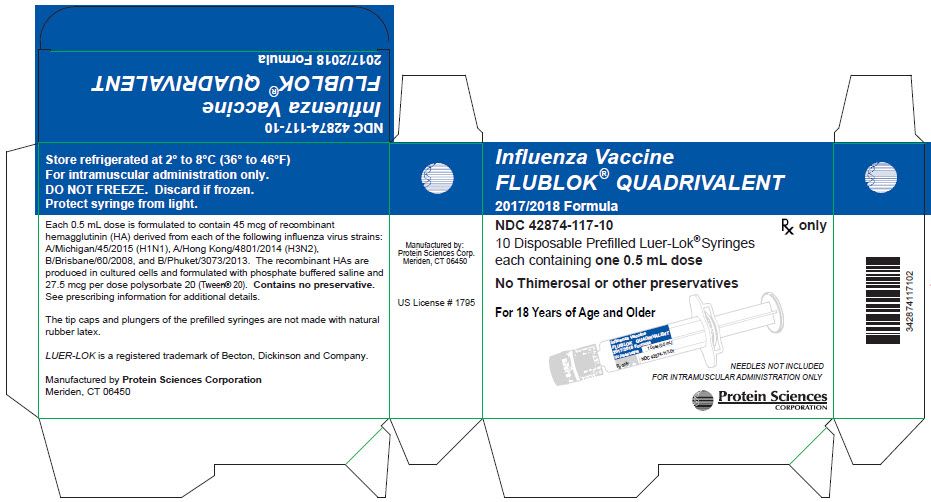 Carton Label
Carton Label
Influenza Vaccine
FLUBLOK® QUADRIVALENT
2017/2018 Formula
NDC 42874-117-10
Rx only
10 Disposable Prefilled Luer-Lok® Syringes
each containing one 0.5 mL dose
No Thimerosal or other preservatives
For 18 Years of Age and Older
NEEDLES NOT INCLUDED
FOR INTRAMUSCULAR ADMINISTRATION ONLY
Protein Sciences Corporation
Store refrigerated at 2° to 8°C (36° to 46°F)
For intramuscular administration only.
DO NOT FREEZE. Discard if frozen.
Protect syringe from light.
Each 0.5 mL dose is formulated to contain 45 mcg of recombinant
hemagglutinin (HA) derived from each of the following influenza virus strains:
A/Michigan/45/2015 (H1N1), A/Hong Kong/4801/2014 (H3N2),
B/Brisbane/60/2008, and B/Phuket/3073/2013. The recombinant HAs are
produced in cultured cells and formulated with phosphate buffered saline and
27.5 mcg per dose polysorbate 20 (Tween® 20). Contains no preservative.
See prescribing information for additional details.
The tip caps and plungers of the prefilled syringes are not made with natural
rubber latex.
LUER-LOK is a registered trademark of Becton, Dickinson and Company.
Manufactured by Protein Sciences Corporation
Meriden, CT 06450
US License # 1795
PRINCIPAL DISPLAY PANEL
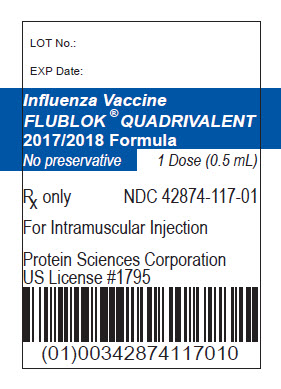 Vial Label
Vial Label
LOT No.:
EXP Date:
Influenza Vaccine
FLUBLOK® QUADRIVALENT
2017-2018 Formula
No Preservative
1 Dose (0.5 mL)
Rx only
NDC 42874-117-01
For Intramuscular Injection
Protein Sciences Corporation
US License # 1795
(01)00342874117010









