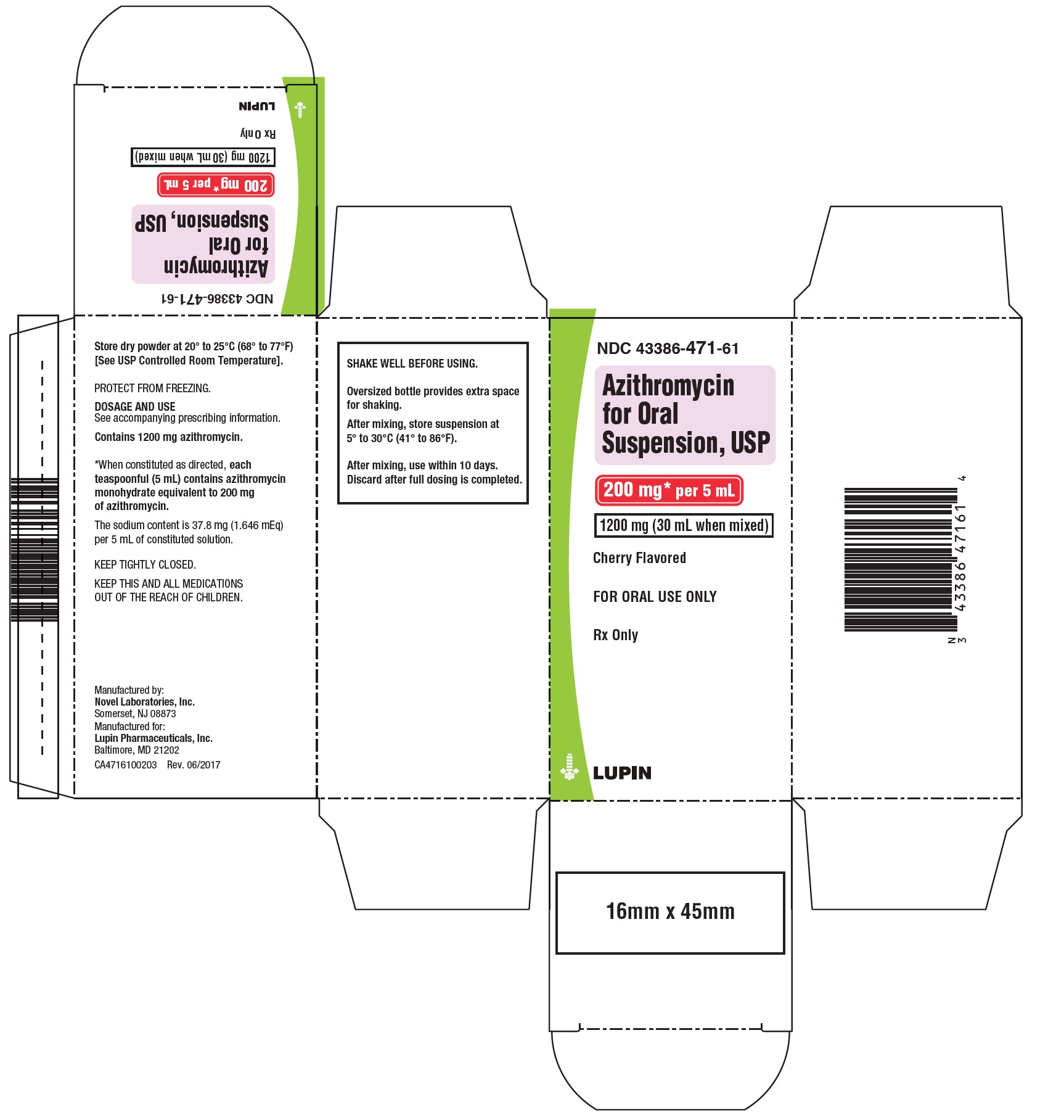
NDC Code(s) : 43386-471-61, 43386-470-60
Packager : Lupin Pharmaceuticals,Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| AzithromycinAzithromycin FOR SUSPENSION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| AzithromycinAzithromycin FOR SUSPENSION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Lupin Pharmaceuticals,Inc.(089153071) |
| REGISTRANT - Novel Laboratories, Inc.(793518643) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Novel Laboratories, Inc. | 793518643 | MANUFACTURE(43386-470, 43386-471), ANALYSIS(43386-470, 43386-471) | |
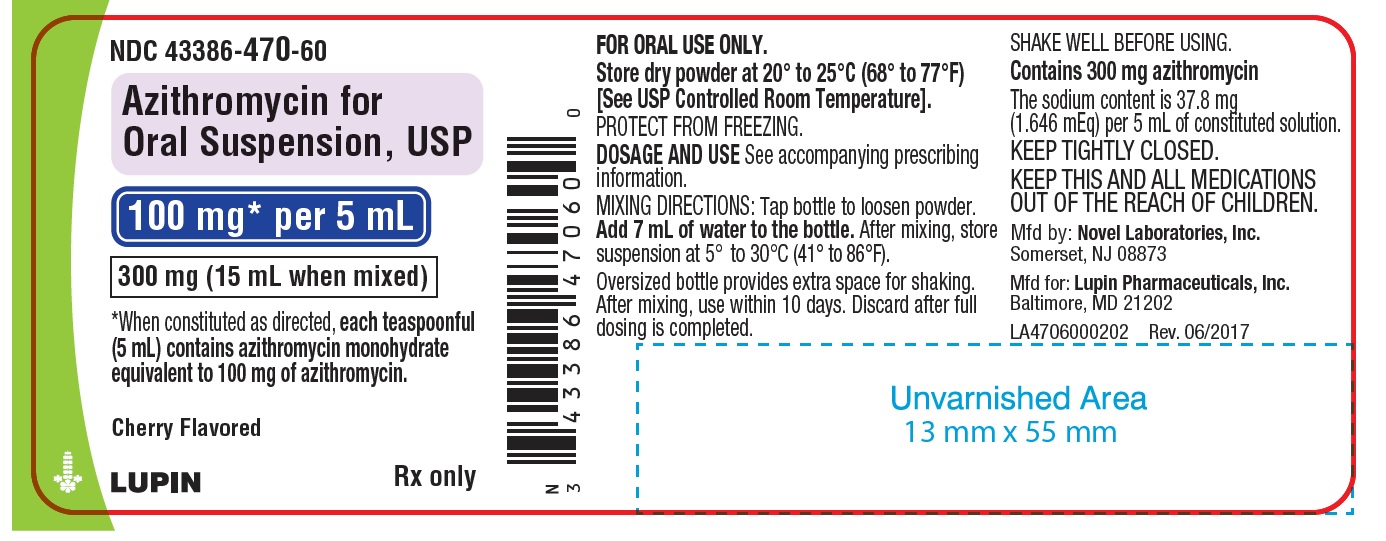
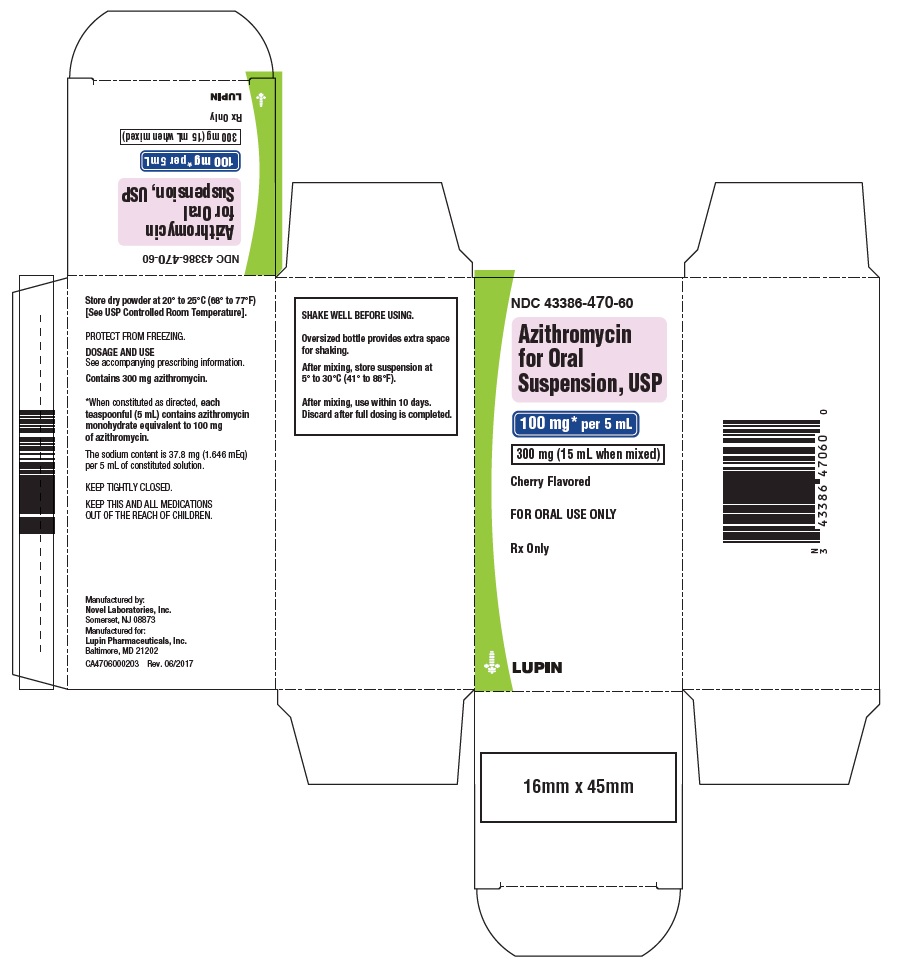
PRINCIPAL DISPLAY PANEL
Azithromycin for Oral Suspension, 100 mg/5 mL
Container Label
NDC: 43386-470-60
Carton Label
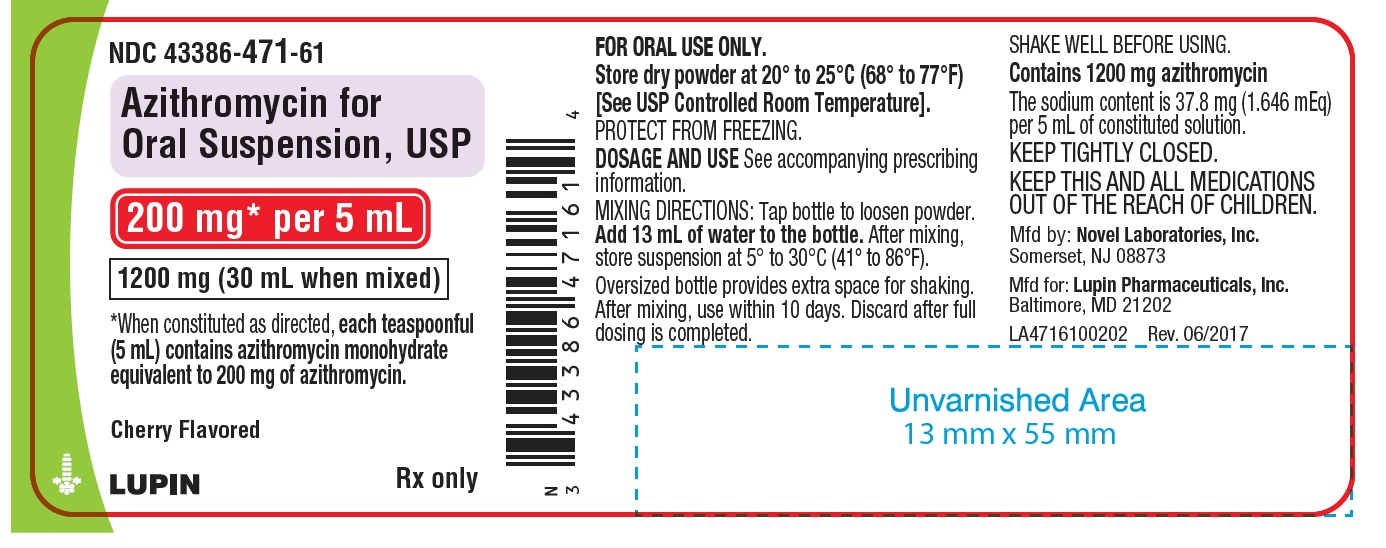
Azithromycin for Oral Suspension, 200 mg/5 mL
1200 mg- Container Label
NDC 43386-471-61
Carton Label