NDC Code(s) : 43419-388-06, 43419-387-06
Packager : AMOREPACIFIC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| IOPE PERFECT SKIN COVER CAKE OCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IOPE PERFECT SKIN COVER CAKE OCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
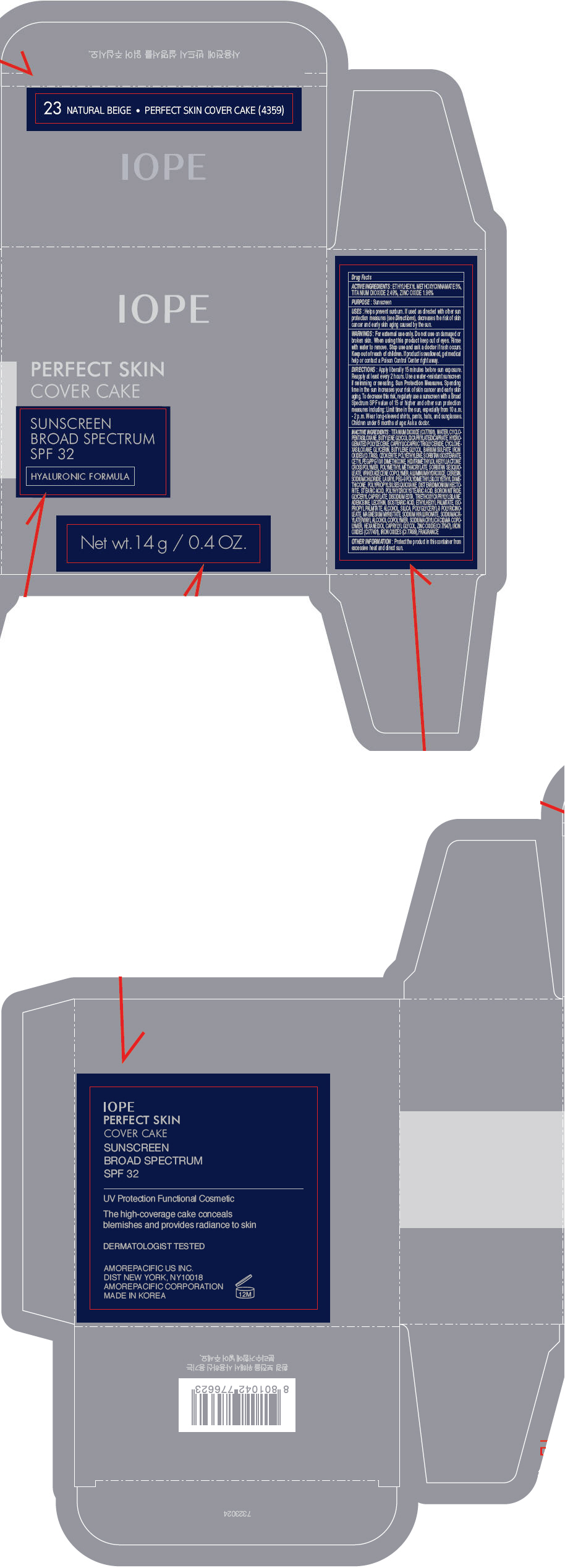
PRINCIPAL DISPLAY PANEL
IOPE
PERFECT SKIN
COVER CAKE
SUNSCREEN
BROAD SPECTRUM
SPF 32
HYALURONIC FORMULA
Net wt. 14 g/0.4 OZ.
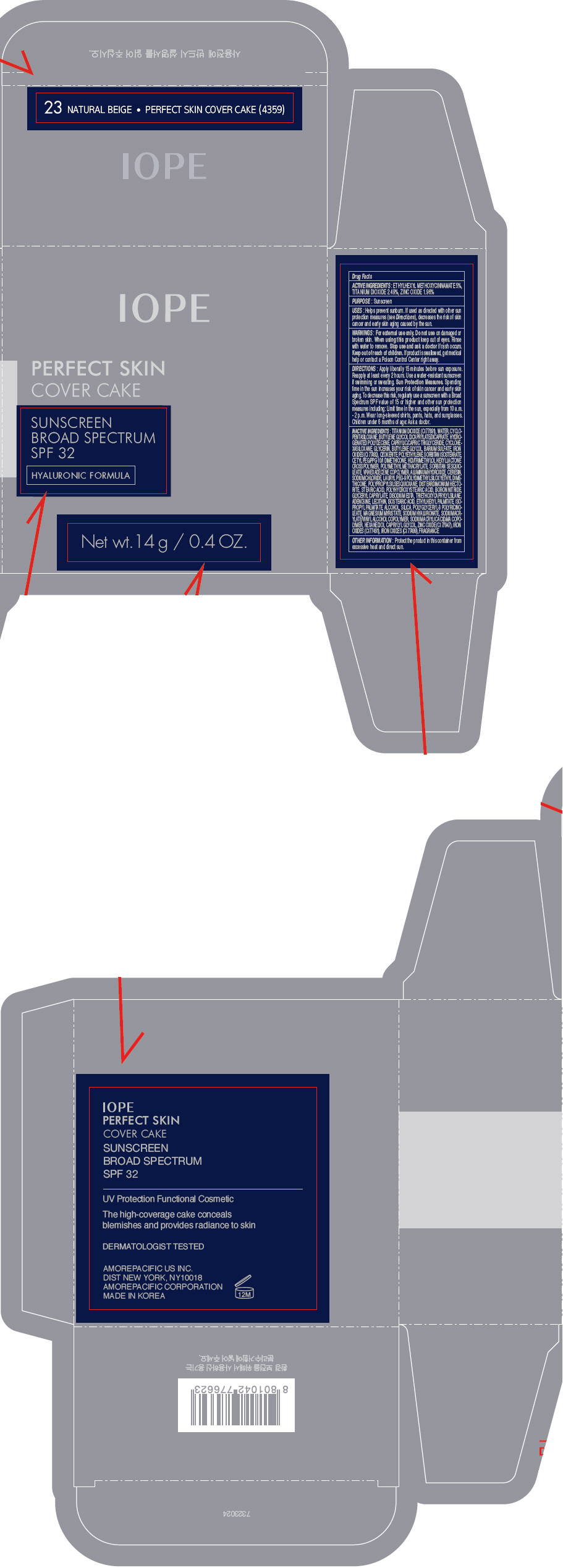
PRINCIPAL DISPLAY PANEL
IOPE
PERFECT SKIN
COVER CAKE
SUNSCREEN
BROAD SPECTRUM
SPF 32
HYALURONIC FORMULA
Net wt. 14 g/0.4 OZ.