NDC Code(s) : 43419-409-03, 43419-410-03
Packager : AMOREPACIFIC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| IOPE SUPER VITAL EXTRA MOIST FOUNDATION No.21OCTINOXATE and TITANIUM DIOXIDE LOTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
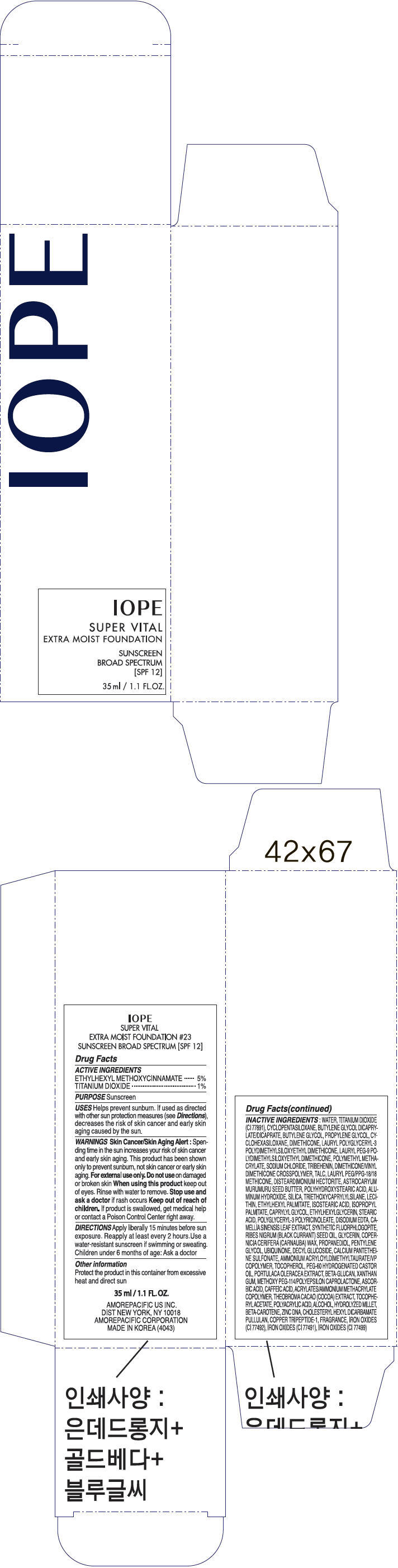
| IOPE SUPER VITAL EXTRA MOIST FOUNDATION No.23OCTINOXATE and TITANIUM DIOXIDE LOTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
IOPE
IOPE
SUPER VITAL
EXTRA MOIST FOUNDATION
SUNSCREEN
BROAD SPECTRUM
[SPF 12]
35 ml / 1.1 FL.OZ.
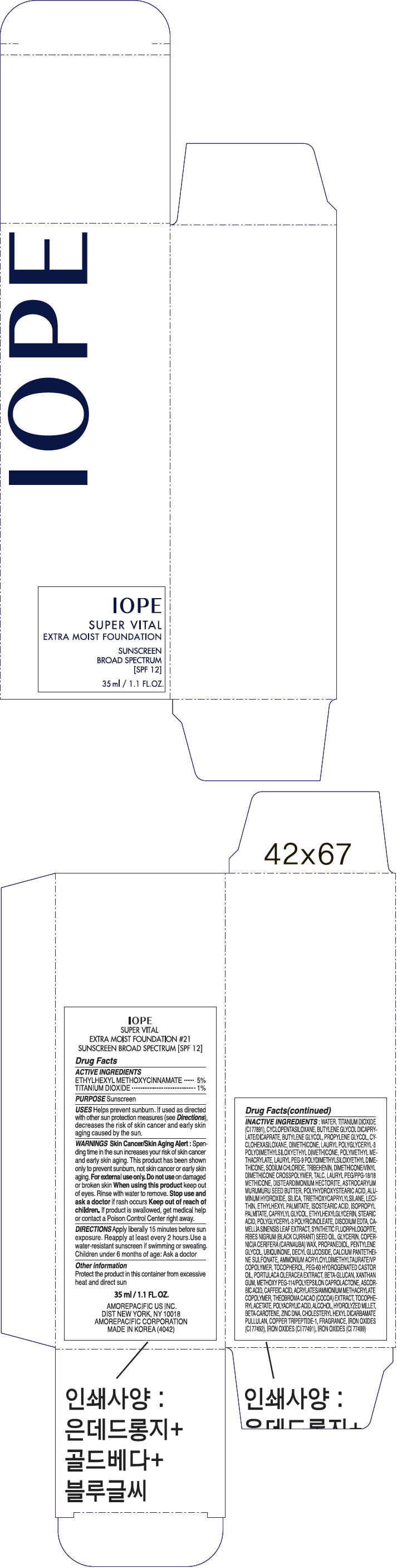
PRINCIPAL DISPLAY PANEL
IOPE
IOPE
SUPER VITAL
EXTRA MOIST FOUNDATION
SUNSCREEN
BROAD SPECTRUM
[SPF 12]
35 ml / 1.1 FL.OZ.