NDC Code(s) : 43419-512-21, 43419-514-21, 43419-511-21, 43419-513-21
Packager : AMOREPACIFIC CORPORATION
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| AMOREPACIFIC OCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE LOTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AMOREPACIFIC OCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE LOTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AMOREPACIFIC OCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE LOTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AMOREPACIFIC OCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE LOTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - AMOREPACIFIC CORPORATION(631035289) |
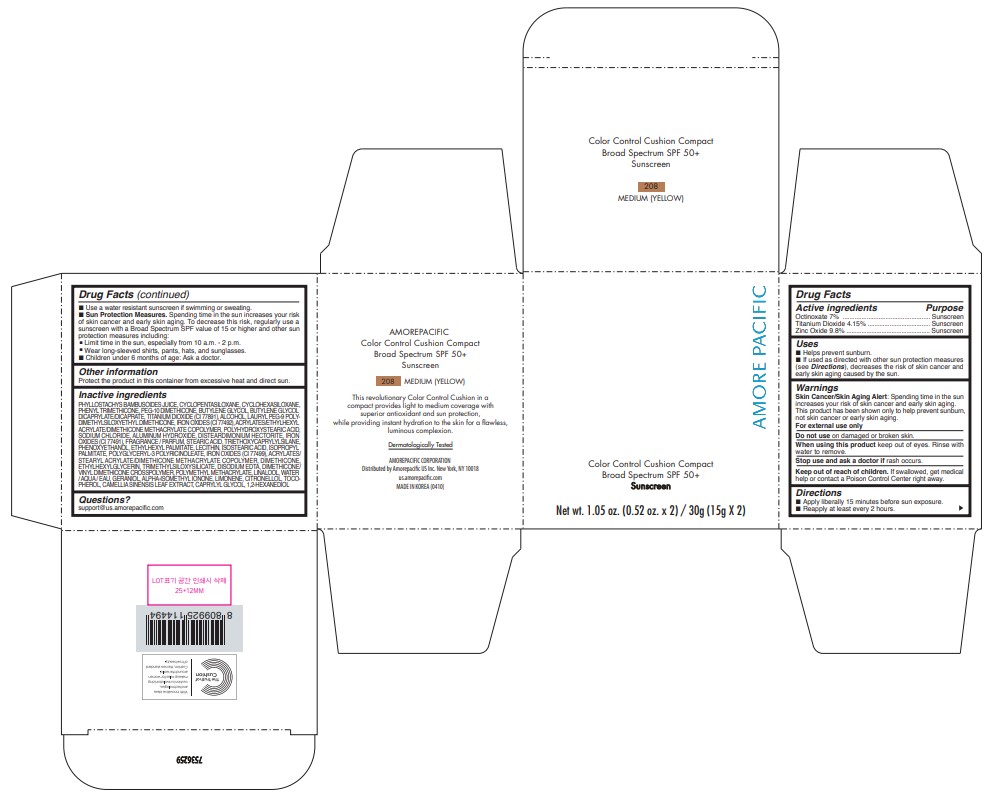
PRINCIPAL DISPLAY PANEL
Amore Pacific
Color Control Cushion Compact
Broad Spectrum SPF 50+
Sunscreen
Net wt. 1.05 oz. (0.52 oz. x 2) / 30g (15g X 2)
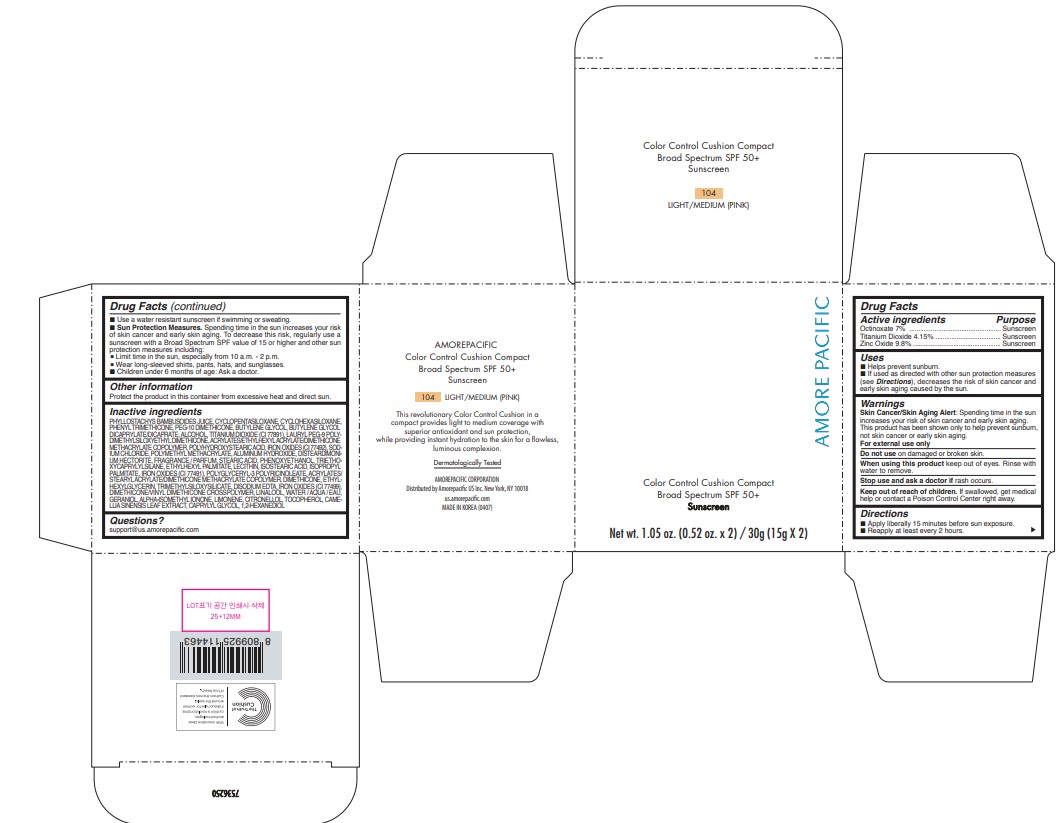
PRINCIPAL DISPLAY PANEL
AMORE PACIFIC
COLOR CONTROL CUSHION COMPACT
Broad Spectrum SPF 50+
Sunscreen
Net wt. 1.05 oz. (0.52 oz. x 2) / 30g (15g X 2)
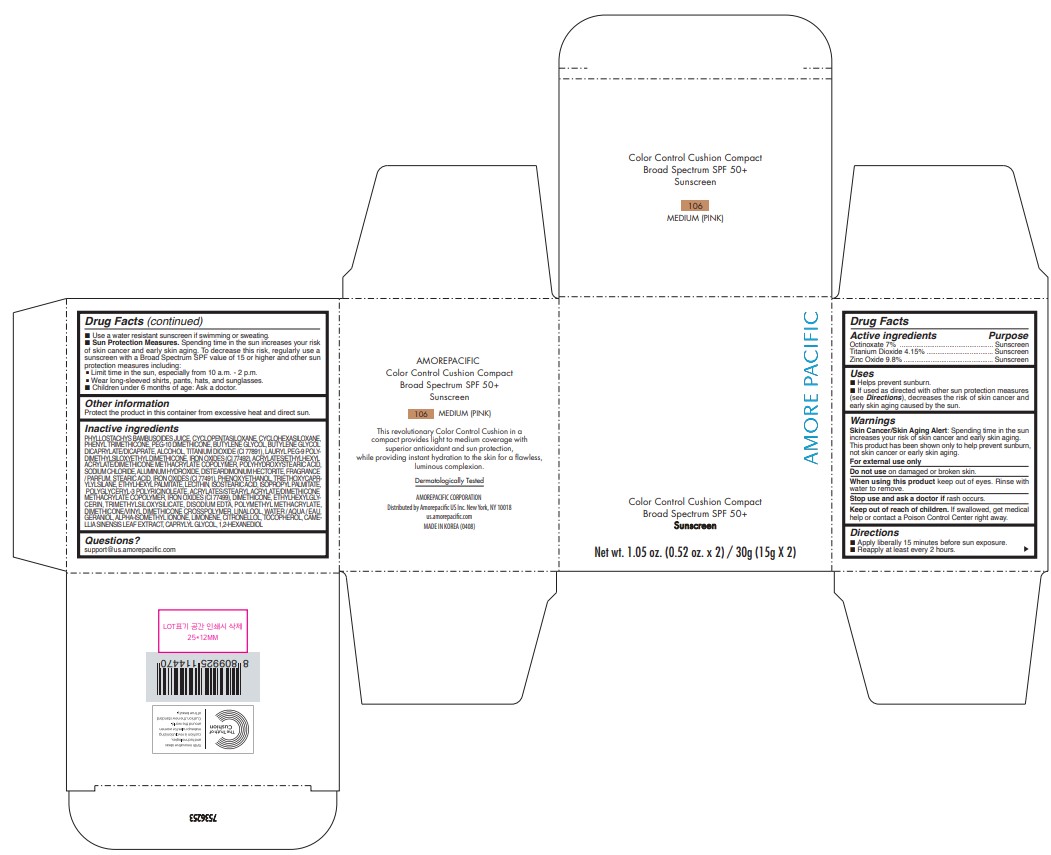
PRINCIPAL DISPLAY PANEL
AMORE PACIFIC
COLOR CONTROL CUSHION COMPACT
Broad Spectrum SPF 50+
Sunscreen
Net wt. 1.05 oz. (0.52 oz. x 2) / 30g (15g X 2)
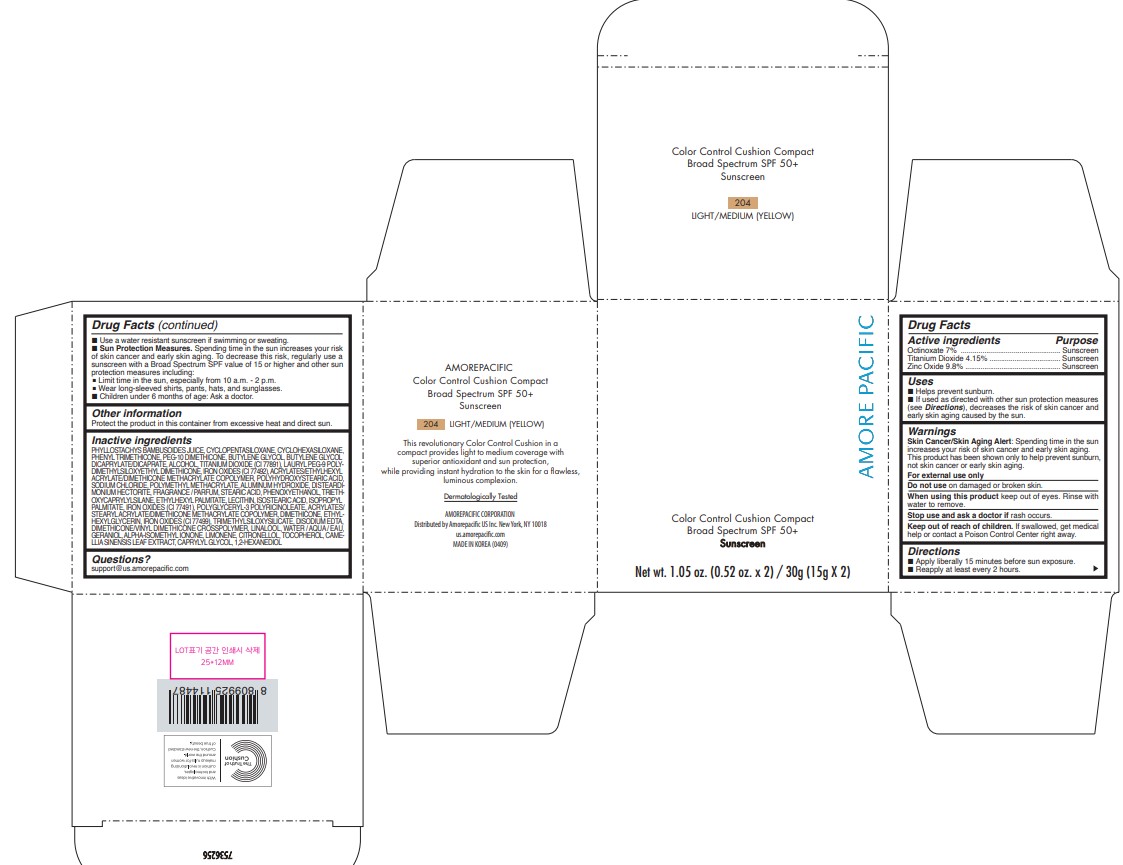
PRINCIPAL DISPLAY PANEL
AMOREPACIFIC
COLOR CONTROL CUSHION COMPACT
Broad Spectrum SPF 50+
Sunscreen
Net wt. 1.05 oz. (0.52 oz. x 2) / 30g (15g X 2)