NDC Code(s) : 43419-707-21, 43419-708-21
Packager : AMOREPACIFIC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
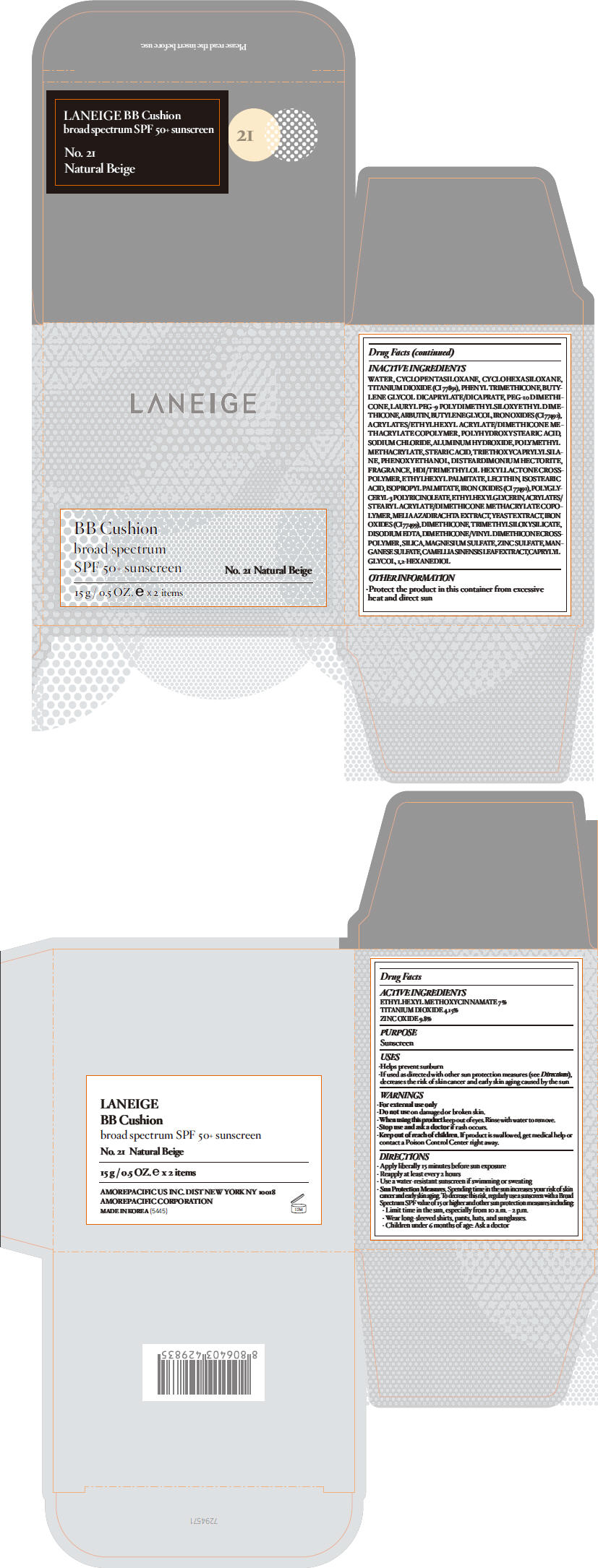
| LANEIGE BB Cushion no.21OCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE LOTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
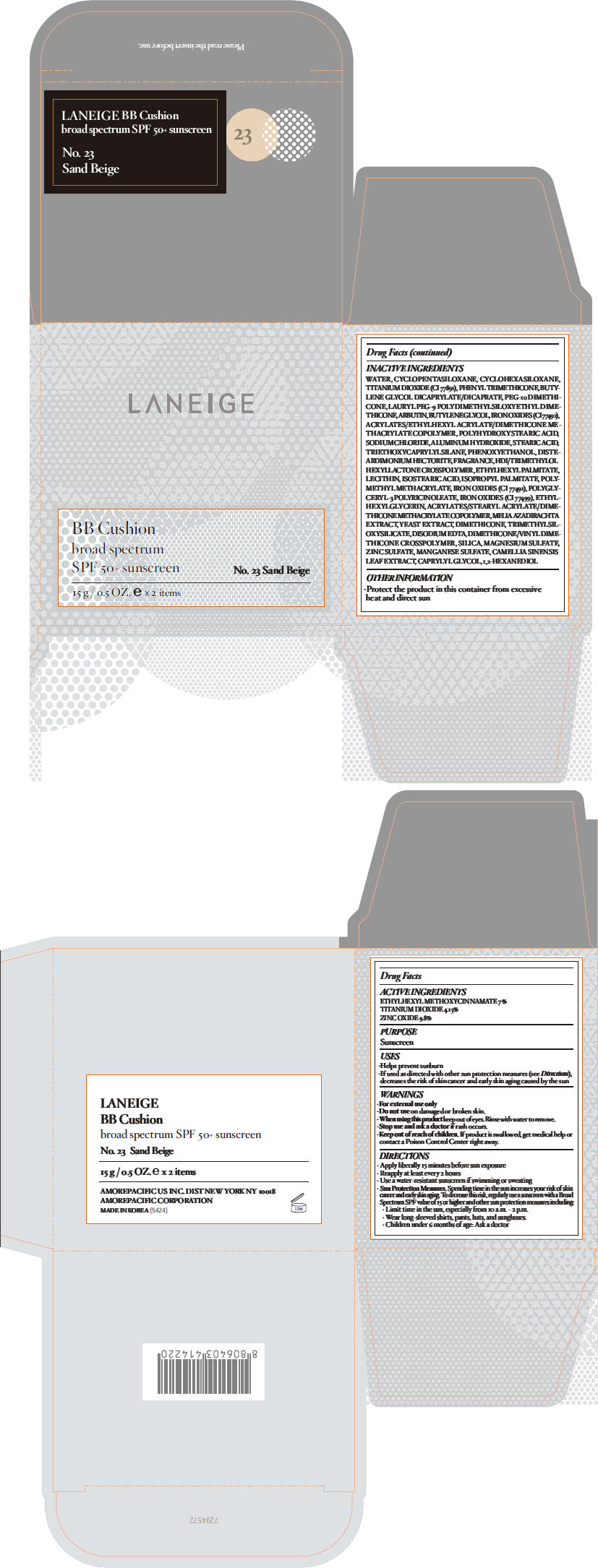
| LANEIGE BB Cushion no.23OCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE LOTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
LANEIGE
BB Cushion
broad spectrum
SPF 50+ sunscreen
No. 21 Natural Beige
15 g / 0.5 OZ. e x 2 items
PRINCIPAL DISPLAY PANEL
LANEIGE
BB Cushion
broad spectrum
SPF 50+ sunscreen
No. 23 Sand Beige
15 g / 0.5 OZ. e x 2 items