NDC Code(s) : 43419-724-29
Packager : AMOREPACIFIC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Laneige Cushion Concealer LightOCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE LOTION | ||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
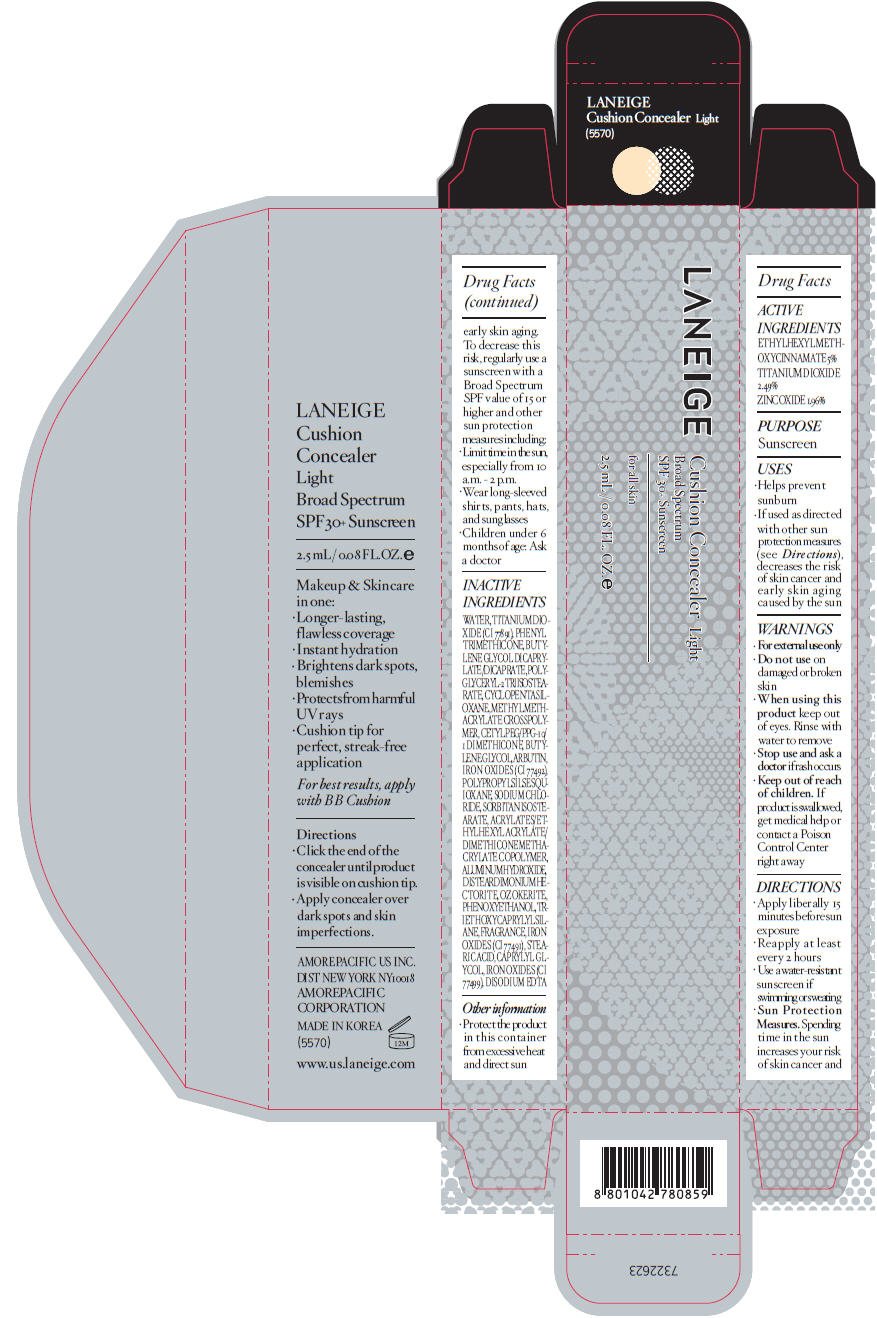
PRINCIPAL DISPLAY PANEL
LANEIGE
Cushion Concealer Light
Broad Spectrum
SPF 30+ sunscreen
for all skin
2.5 mL / 0.08 FL. OZ. e