NDC Code(s) : 43419-761-31, 43419-762-31, 43419-763-31, 43419-764-31, 43419-766-31, 43419-767-31
Packager : Amorepacific Corporation
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| LANEIGE BB CUSHION HYDRA RADIANCE Limited Edition with Crystals No.11 PorcelainZINC OXIDE, OCTINOXATE, and TITANIUM DIOXIDE LOTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LANEIGE BB CUSHION HYDRA RADIANCE Limited Edition with Crystals No.21 BeigeZINC OXIDE, OCTINOXATE, and TITANIUM DIOXIDE LOTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LANEIGE BB CUSHION HYDRA RADIANCE Limited Edition with Crystals No.23 SandZINC OXIDE, OCTINOXATE, and TITANIUM DIOXIDE LOTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LANEIGE BB CUSHION HYDRA RADIANCE Limited Edition with Crystals No.33 CinnamonZINC OXIDE, OCTINOXATE, and TITANIUM DIOXIDE LOTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LANEIGE BB CUSHION HYDRA RADIANCE Limited Edition with Crystals No.35 CoffeeZINC OXIDE, OCTINOXATE, and TITANIUM DIOXIDE LOTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LANEIGE BB CUSHION HYDRA RADIANCE Limited Edition with Crystals No.37 CacaoZINC OXIDE, OCTINOXATE, and TITANIUM DIOXIDE LOTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
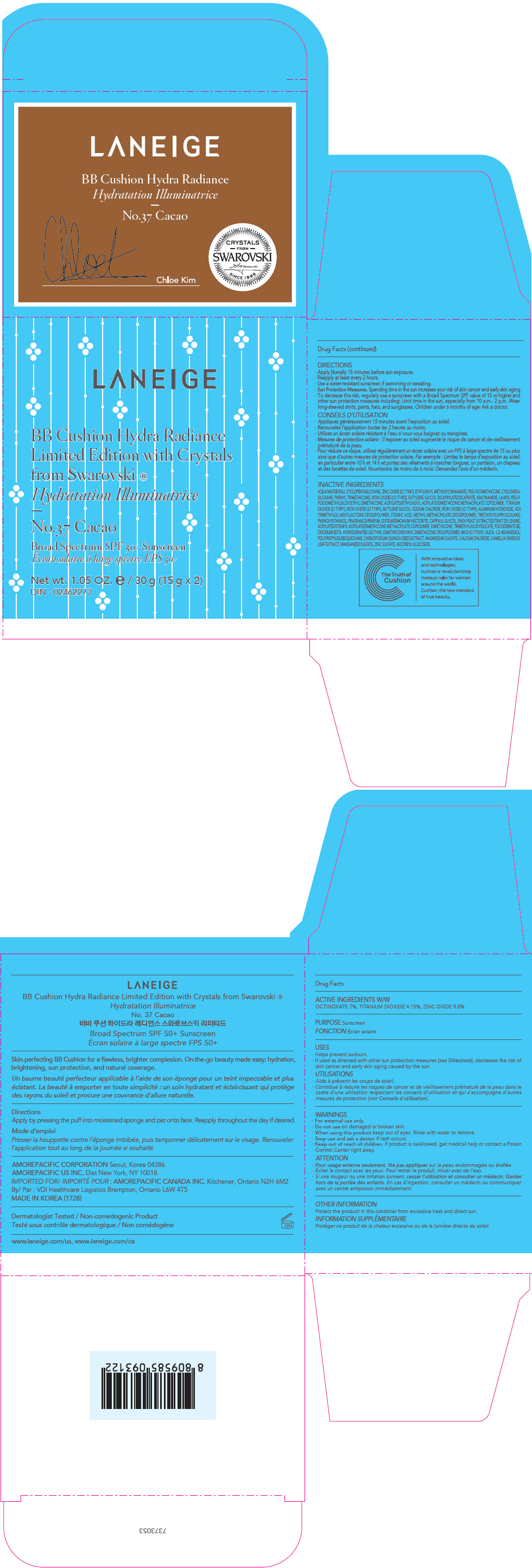
PRINCIPAL DISPLAY PANEL
LANEIGE
BB Cushion Hydra Radiance
Limited Edition with Crystals
from Swarovski ®
No.11 Porcelain
Broad Spectrum SPF 50+ Sunscreen
Net wt. 1.05 OZ. e / 30 g (15 g x 2)
DIN : 02462273
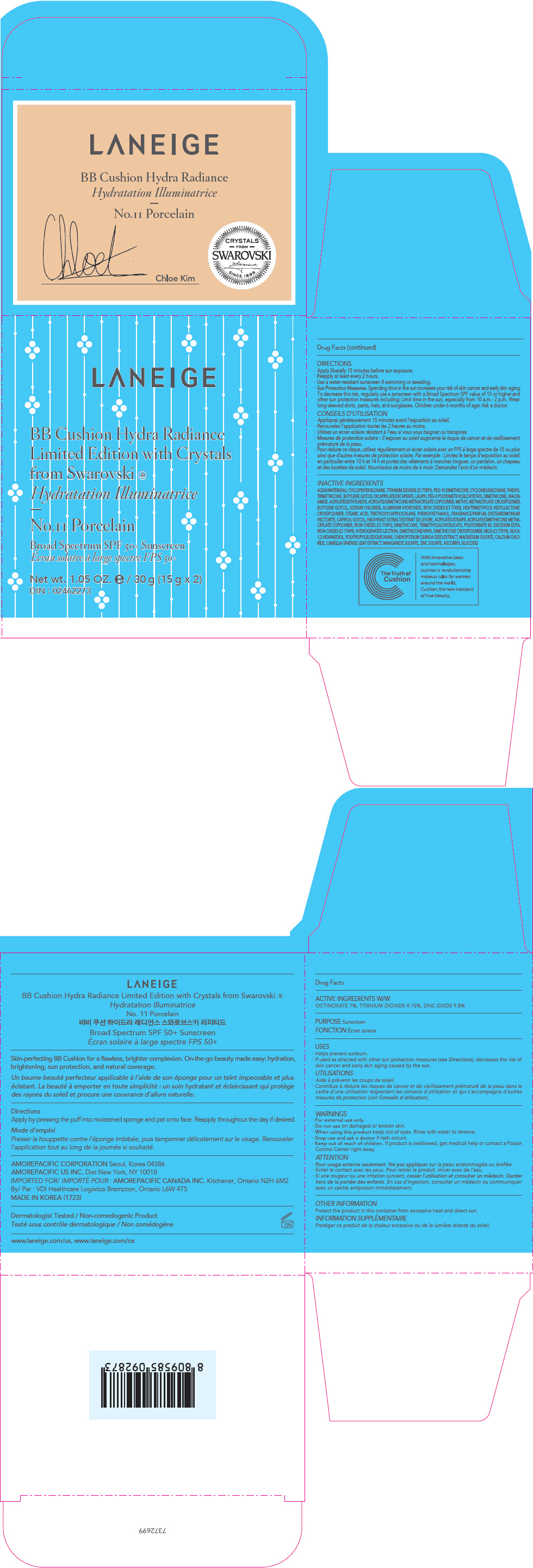
PRINCIPAL DISPLAY PANEL
LANEIGE
BB Cushion Hydra Radiance
Limited Edition with Crystals
from Swarovski ®
No.21 Beige
Broad Spectrum SPF 50+ Sunscreen
Net wt. 1.05 OZ. e / 30 g (15 g x 2)
DIN : 02462273
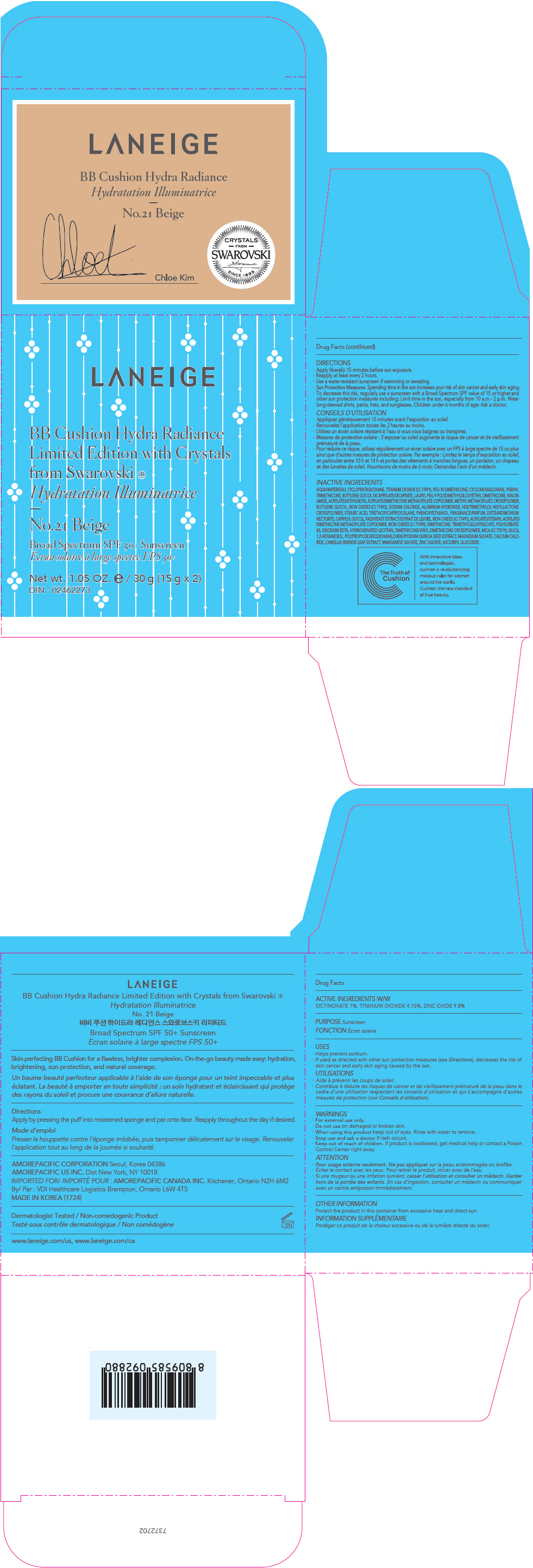
PRINCIPAL DISPLAY PANEL
LANEIGE
BB Cushion Hydra Radiance
Limited Edition with Crystals
from Swarovski ®
No.23 Sand
Broad Spectrum SPF 50+ Sunscreen
Net wt. 1.05 OZ. e / 30 g (15 g x 2)
DIN : 02462273
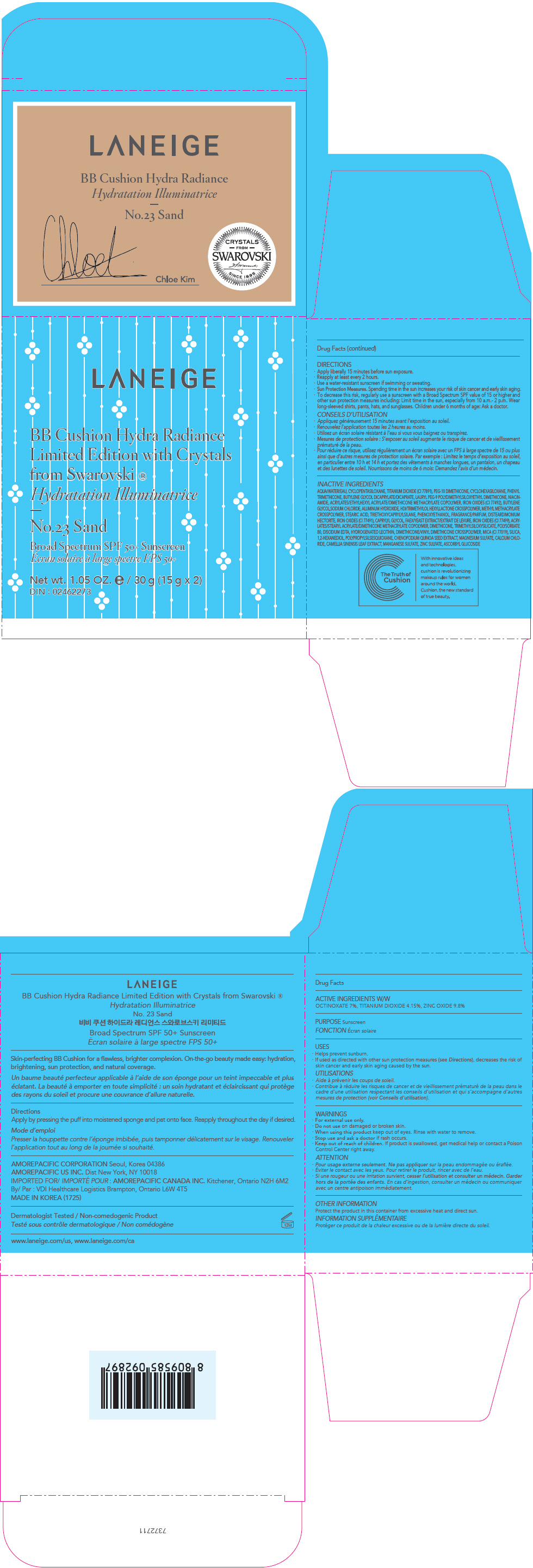
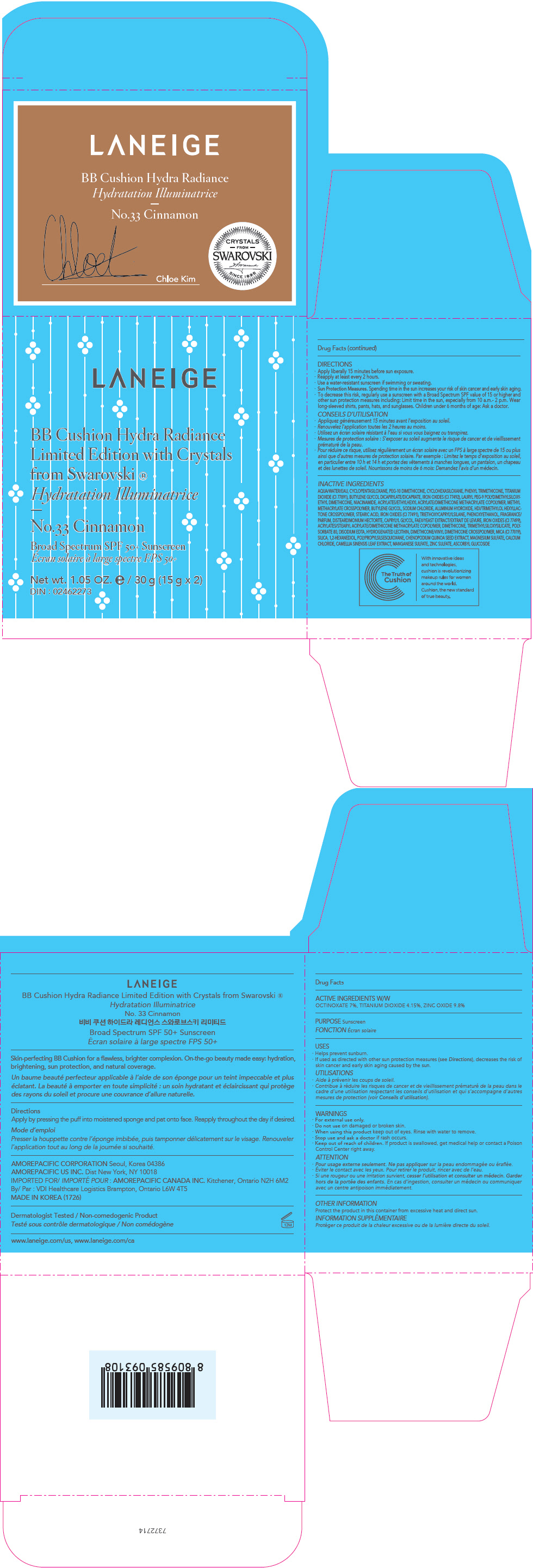
PRINCIPAL DISPLAY PANEL
LANEIGE
BB Cushion Hydra Radiance
Limited Edition with Crystals
from Swarovski ®
No.33 Cinnamon
Broad Spectrum SPF 50+ Sunscreen
Net wt. 1.05 OZ. e / 30 g (15 g x 2)
DIN : 02462273
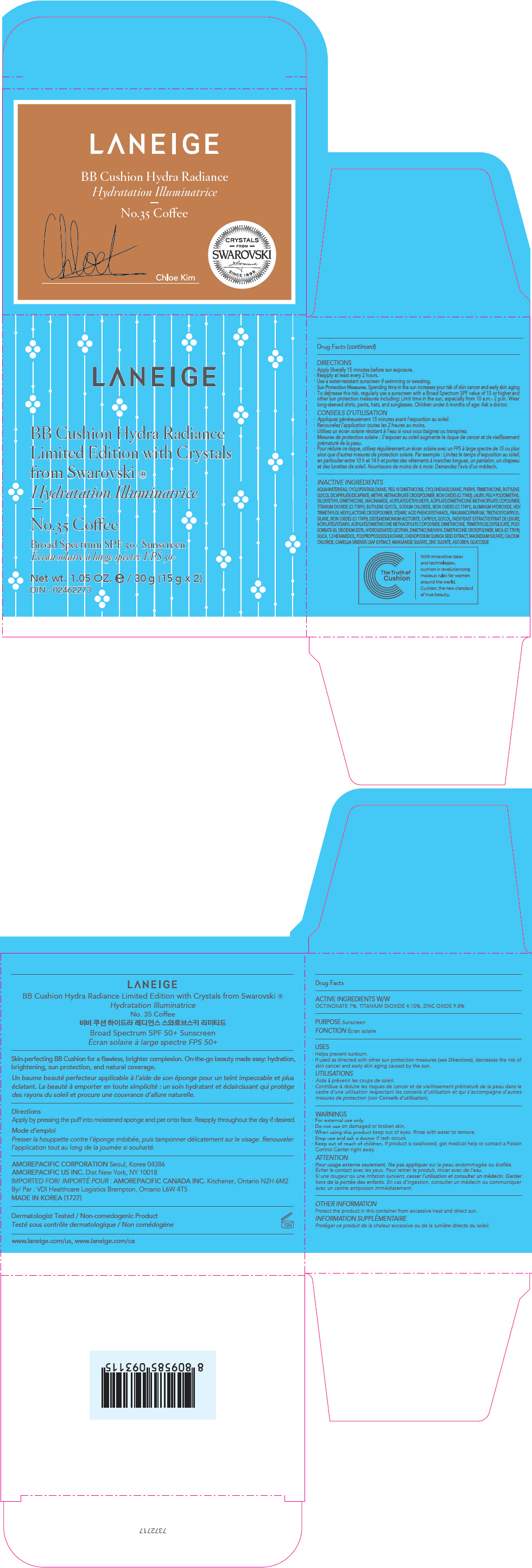
PRINCIPAL DISPLAY PANEL
LANEIGE
BB Cushion Hydra Radiance
Limited Edition with Crystals
from Swarovski ®
No.35 Coffee
Broad Spectrum SPF 50+ Sunscreen
Net wt. 1.05 OZ. e / 30 g (15 g x 2)
DIN : 02462273
PRINCIPAL DISPLAY PANEL
LANEIGE
BB Cushion Hydra Radiance
Limited Edition with Crystals
from Swarovski ®
No.37 Cacao
Broad Spectrum SPF 50+ Sunscreen
Net wt. 1.05 OZ. e / 30 g (15 g x 2)
DIN : 02462273