NDC Code(s) : 43547-524-03, 43547-524-09, 43547-524-50, 43547-525-03, 43547-525-09, 43547-525-50, 43547-526-03, 43547-526-09, 43547-526-50, 43547-527-03, 43547-527-09, 43547-527-50
Packager : Solco Healthcare US, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| NEBIVOLOLnebivolol TABLET | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| NEBIVOLOLnebivolol TABLET | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| NEBIVOLOLnebibolol TABLET | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| NEBIVOLOLnebivolol TABLET | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Solco Healthcare US, LLC(828343017) |
| REGISTRANT - Prinston Pharmaceutical Inc.(967289799) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Zhejiang Huahai Pharmaceutical Co., LTD | 530732460 | MANUFACTURE(43547-524, 43547-525, 43547-526, 43547-527) | |
PRINCIPAL DISPLAY PANEL
Container Label-2.5 mg-30 tablets
Rx only
NDC 43547-524-03
Nebivolol Tablets
Each tablet contains: nebivolol hydrochloride equivalent to 2.5 mg nebivolol.
This package is child-resistant. Keep this and all drugs out of the reach of children.
Dispense in tight, light-resistant container as defined in the USP, using a child-resistant closure.
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° and 30°C ° (59 and 86°F). [See USP for Controlled Room Temperature.]
See package insert for dosing and full Prescribing Information.
Manufactured by:
Zhejiang Huahai Pharmaceutical Co., Ltd.
Xunqiao, Linhai, Zhejiang 317024, China
Distributed by:
Solco Healthcare US, LLC
Somerset, NJ 08873, USA
Rev.: 02/2022

PRINCIPAL DISPLAY PANEL
Container Label-5 mg-30 tablets
Rx only
NDC 43547-525-03
Nebivolol Tablets
Each tablet contains: nebivolol hydrochloride equivalent to 5 mg nebivolol.
This package is child-resistant. Keep this and all drugs out of the reach of children.
Dispense in tight, light-resistant container as defined in the USP, using a child-resistant closure.
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° and 30°C ° (59 and 86°F). [See USP for Controlled Room Temperature.]
See package insert for dosing and full Prescribing Information.
Manufactured by:
Zhejiang Huahai Pharmaceutical Co., Ltd.
Xunqiao, Linhai, Zhejiang 317024, China
Distributed by:
Solco Healthcare US, LLC
Somerset, NJ 08873, USA
Rev.: 02/2022

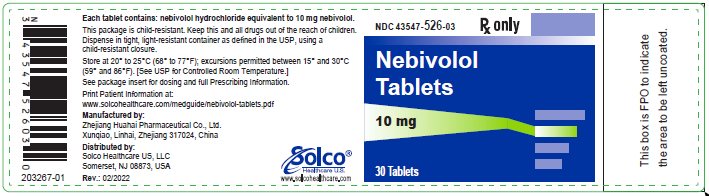
PRINCIPAL DISPLAY PANEL
Container Label-10 mg-30 tablets
Rx only
NDC 43547-526-03
Nebivolol Tablets
Each tablet contains: nebivolol hydrochloride equivalent to 10 mg nebivolol.
This package is child-resistant. Keep this and all drugs out of the reach of children.
Dispense in tight, light-resistant container as defined in the USP, using a child-resistant closure.
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° and 30°C ° (59 and 86°F). [See USP for Controlled Room Temperature.]
See package insert for dosing and full Prescribing Information.
Manufactured by:
Zhejiang Huahai Pharmaceutical Co., Ltd.
Xunqiao, Linhai, Zhejiang 317024, China
Distributed by:
Solco Healthcare US, LLC
Somerset, NJ 08873, USA
Rev.: 02/2022
PRINCIPAL DISPLAY PANEL
Container Label-20 mg-30 tablets
Rx only
NDC 43547-527-03
Nebivolol Tablets
Each tablet contains: nebivolol hydrochloride equivalent to 20 mg nebivolol.
This package is child-resistant. Keep this and all drugs out of the reach of children.
Dispense in tight, light-resistant container as defined in the USP, using a child-resistant closure.
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° and 30°C ° (59 and 86°F). [See USP for Controlled Room Temperature.]
See package insert for dosing and full Prescribing Information.
Manufactured by:
Zhejiang Huahai Pharmaceutical Co., Ltd.
Xunqiao, Linhai, Zhejiang 317024, China
Distributed by:
Solco Healthcare US, LLC
Somerset, NJ 08873, USA
Rev.: 02/2022










